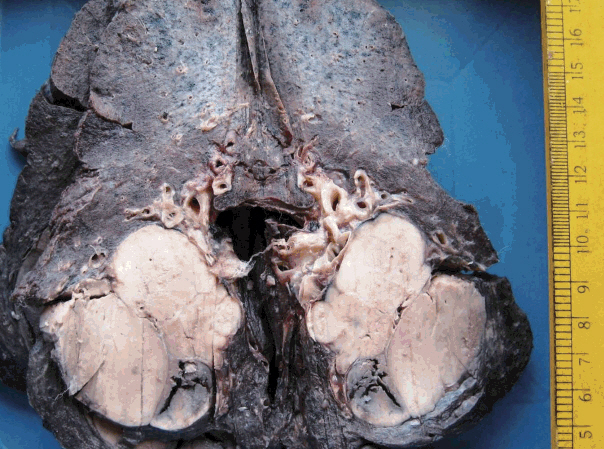
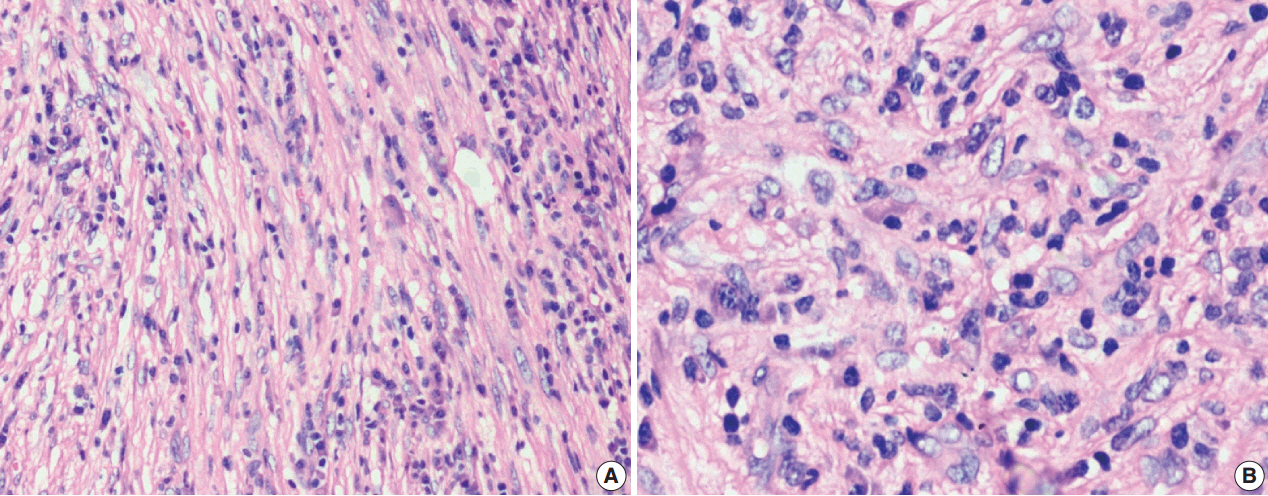
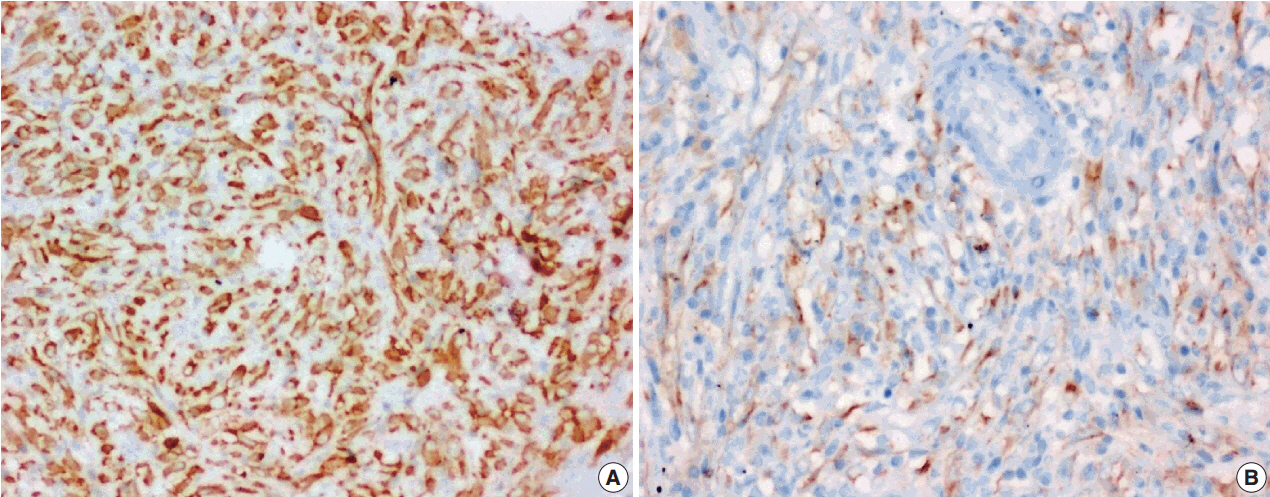
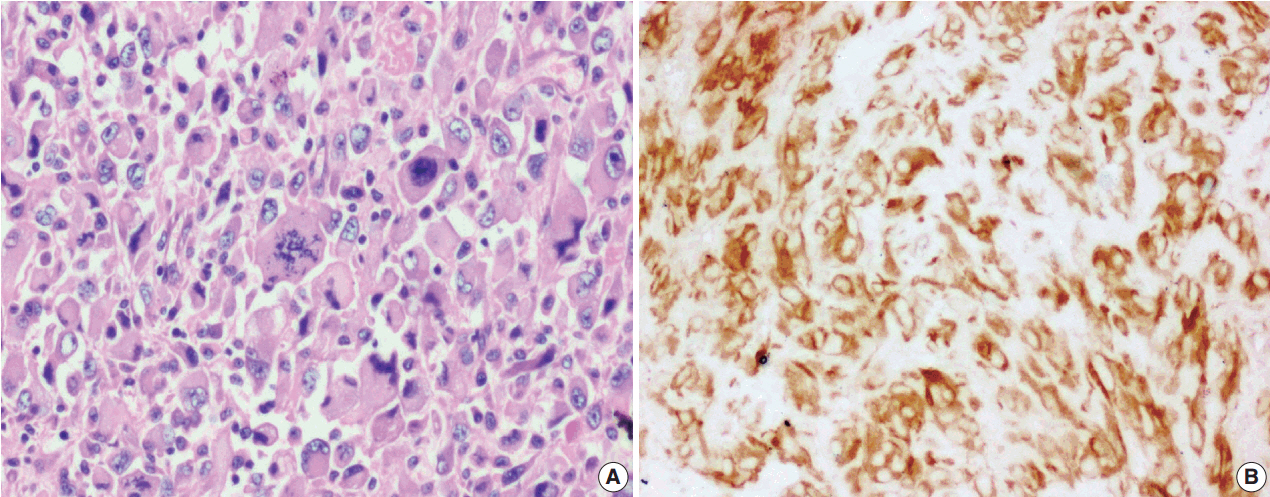
J Pathol Transl Med.
2017 May;51(3):255-263. 10.4132/jptm.2017.01.12.
Clinicopathological Study of 18 Cases of Inflammatory Myofibroblastic Tumors with Reference to ALK-1 Expression: 5-Year Experience in a Tertiary Care Center
- Affiliations
-
- 1Department of General Pathology, Christian Medical College and Hospital, Vellore, India. dr.rameshtelugu@gmail.com
- 2Department of Paediatric Surgery, Christian Medical College and Hospital, Vellore, India.
- 3Department of Thoracic Surgery, Christian Medical College and Hospital, Vellore, India.
- KMID: 2392593
- DOI: http://doi.org/10.4132/jptm.2017.01.12
Abstract
- BACKGROUND
Inflammatory myofibroblastic tumor is a histopathologically distinctive neoplasm of children and young adults. According to World Health Organization (WHO) classification, inflammatory myofibroblastic tumor is an intermediate-grade tumor, with potential for recurrence and rare metastasis. There are no definite histopathologic, molecular, or cytogenetic features to predict malignant transformation, recurrence, or metastasis.
METHODS
A 5-year retrospective study of histopathologically diagnosed inflammatory myofibroblastic tumors of various anatomic sites was conducted to correlate anaplastic lymphoma kinase-1 (ALK-1) expression with histological atypia, multicentric origin of tumor, recurrence, and metastasis. Clinical details of all the cases were noted from the clinical work station. Immunohistochemical stains for ALK-1 and other antibodies were performed. Statistical analysis was done using Fisher exact test.
RESULTS
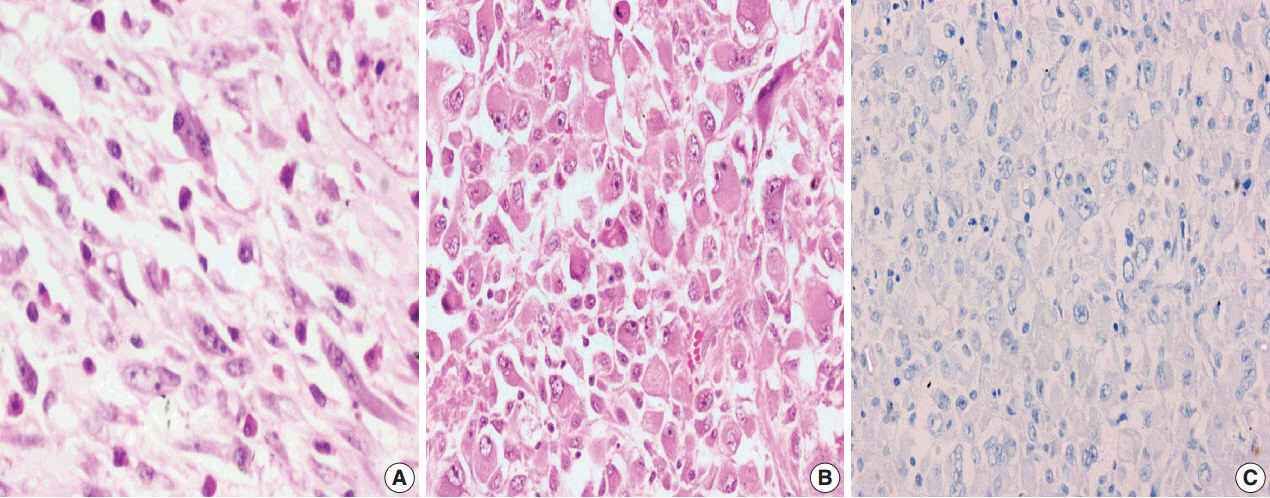
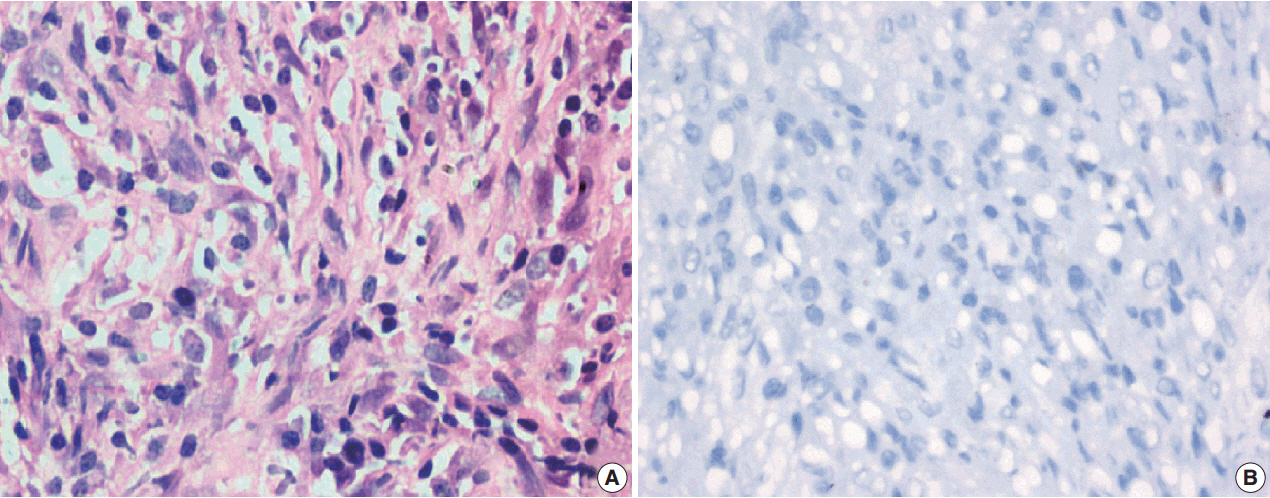
A total of 18 cases of inflammatory myofibroblastic tumors were found during the study period, of which 14 were classical. The female-male ratio was 1:1 and the mean age was 23.8 years. Histologically atypical (four cases) and multifocal tumors (three cases, multicentric in origin) were noted. Recurrence was noted in 30% of ALK-1 positive and 37.5% of ALK-1 negative cases, whereas metastasis to the lung, liver, and pelvic bone was noted in the ALK-1 positive group only.
CONCLUSIONS
Overall, ALK-1 protein was expressed in 55.6% of inflammatory myofibroblastic tumors. There was no statistically significant correlation between ALK-1 expression, tumor type, recurrence and metastasis. However, ALK-1 immunohistochemistry is a useful diagnostic aid in the appropriate clinical and histomorphologic context.
Keyword
MeSH Terms
Figure
Reference
-
1. Ufuk F, Herek D, Karabulut N. Inflammatory myofibroblastic tumor of the lung: unusual imaging findings of three cases. Pol J Radiol. 2015; 80:479–82.
Article2. Umiker WO, Iverson L. Postinflammatory tumors of the lung: report of four cases simulating xanthoma, fibroma or plasma cell tumor. J Thorac Surg. 1954; 28:55–63.3. Fletcher CD, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press;2013. p. 83–4.4. Marino-Enriquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: an aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011; 35:135–44.5. Li J, Yin WH, Takeuchi K, Guan H, Huang YH, Chan JK. Inflammatory myofibroblastic tumor with RANBP2 and ALK gene rearrangement: a report of two cases and literature review. Diagn Pathol. 2013; 8:147.
Article6. Savvidou OD, Sakellariou VI, Papakonstantinou O, Skarpidi E, Papagelopoulos PJ. Inflammatory myofibroblastic tumor of the thigh: presentation of a rare case and review of the literature. Case Rep Orthop. 2015; 2015:814241.
Article7. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007; 31:509–20.8. Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: a case report. World J Gastroenterol. 2012; 18:7100–3.
Article9. Wang Z, Zhao X, Li K, et al. Analysis of clinical features and outcomes for inflammatory myofibroblastic tumors in China: 11 years of experience at a single center. Pediatr Surg Int. 2016; 32:239–43.
Article10. Karnak I, Senocak ME, Ciftci AO, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg. 2001; 36:908–12.
Article11. Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg. 2011; 46:2076–82.12. Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999; 12:279–86.13. Jiang YH, Cheng B, Ge MH, Cheng Y, Zhang G. Comparison of the clinical and immunohistochemical features, including anaplastic lymphoma kinase (ALK) and p53, in inflammatory myofibroblastic tumours. J Int Med Res. 2009; 37:867–77.
Article14. Janik JS, Janik JP, Lovell MA, Hendrickson RJ, Bensard DD, Greffe BS. Recurrent inflammatory pseudotumors in children. J Pediatr Surg. 2003; 38:1491–5.
Article15. Chaudhary P. Mesenteric inflammatory myofibroblastic tumors. Ann Gastroenterol. 2015; 28:49–54.16. Ramotar H, Cheung L, Pitkin L. The great mimicker: a rare case of head and neck inflammatory pseudotumour in the presence of human immunodeficiency virus. J Laryngol Otol. 2016; 130:107–10.
Article17. Li XQ, Hisaoka M, Shi DR, Zhu XZ, Hashimoto H. Expression of anaplastic lymphoma kinase in soft tissue tumors: an immunohistochemical and molecular study of 249 cases. Hum Pathol. 2004; 35:711–21.
Article18. Jacob SV, Reith JD, Kojima AY, Williams WD, Liu C, Vila Duckworth L. An unusual case of systemic inflammatory myofibroblastic tumor with successful treatment with ALK-inhibitor. Case Rep Pathol. 2014; 2014:470340.
Article19. Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010; 363:1727–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Analysis and Treatment of Adult Patients with Inflammatory Myofibroblastic Tumor: A 15-Year Single- Center Study
- Primary epithelioid inflammatory myofibroblastic sarcoma of the brain with EML4::ALK fusion mimicking intra-axial glioma: a case report and brief literature review
- A Case of Simultaneously Diagnosed Lung Adenocarcinoma and Endobronchial Inflammatory Myofibroblastic Tumor with Two Distinct Types of ALK Translocation
- Inflammatory Myofibroblastic Tumor of Nasal Septum after Septoplasty: A Case Report
- Oral spindle cell/sclerosing rhabdomyosarcoma on mandible with anaplastic lymphoma kinase expression mimicking inflammatory myofibroblastic tumor