Ann Surg Treat Res.
2017 Jul;93(1):1-10. 10.4174/astr.2017.93.1.1.
Omega-3 fatty acids inhibit oxidative stress in a rat model of liver regeneration
- Affiliations
-
- 1Department of General Surgery, Ege University Hospital, Izmir, Turkey. ozgur.firat@ege.edu.tr
- 2Department of Pharmacology, Faculty of Pharmacy, Ege University, Izmir, Turkey.
- 3Department of Medical Biochemistry, Adnan Menderes University Hospital, Aydın, Turkey.
- KMID: 2392277
- DOI: http://doi.org/10.4174/astr.2017.93.1.1
Abstract
- PURPOSE
Lipid peroxidation and consequent reactive oxygen species in the setting of oxidative stress have crucial roles in liver regeneration, which may adversely affect the regeneration itself and lead to liver failure. The aim of the current study is to investigate whether omega-3 fatty acid supplementation inhibits oxidative stress in an experimental model of liver regeneration.
METHODS
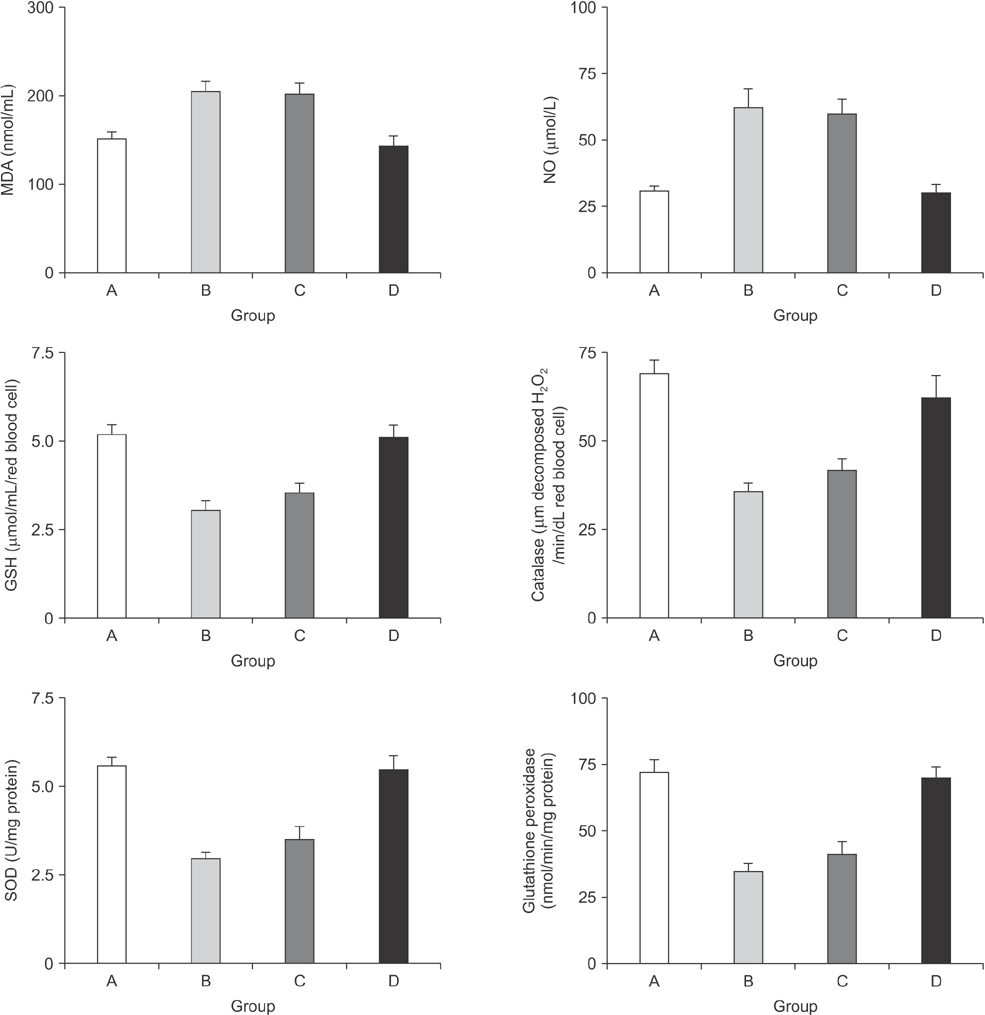
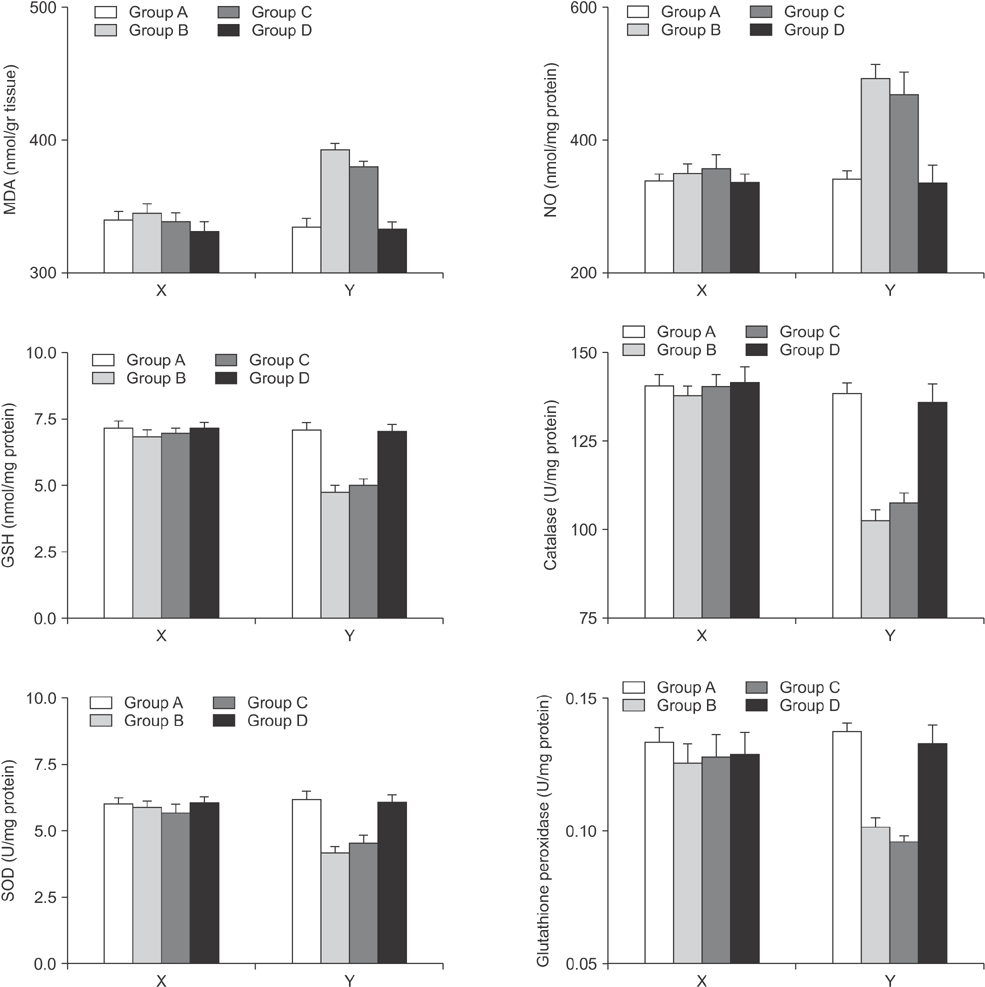
Forty rats were allocated to four groups. Rats in group A received a sham operation. Rats in group B were subjected to right portal vein ligation (RPVL) and saline infusion. Rats in groups C and D were subjected to RPVL and total parenteral nutrition (TPN) with an all-in-one admixture containing a soybean oil based lipid emulsion. Rats in group D were additionally supplemented with omega-3 fatty acid infusion. Oxidative stresses in the blood and liver were measured by glutathione, superoxide dismutase, catalase, glutathione peroxidase, malondialdehyde, and nitric oxide.
RESULTS
Omega-3 supplementation to the TPN solution significantly corrected alterations in the blood and tissue concentrations of oxidants and anti-oxidants during regeneration (P < 0.05).
CONCLUSION
Omega-3 fatty acid supplementation to the TPN solution revealed promising results in removal of oxidative stress that emerges during liver regeneration.
Figure
Cited by 1 articles
-
Effects of hyperbaric oxygen and iloprost on intestinal ischemia-reperfusion induced acute lung injury
Yeliz Yilmaz, Levent Tumkaya
Ann Surg Treat Res. 2019;96(1):34-40. doi: 10.4174/astr.2019.96.1.34.
Reference
-
1. Yang S, Tan TM, Wee A, Leow CK. Mitochondrial respiratory function and antioxidant capacity in normal and cirrhotic livers following partial hepatectomy. Cell Mol Life Sci. 2004; 61:220–229.2. Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004; 39:386–392.3. Hong JY, Lebofsky M, Farhood A, Jaeschke H. Oxidant stress-induced liver injury in vivo: role of apoptosis, oncotic necrosis, and c-Jun NH2-terminal kinase activation. Am J Physiol Gastrointest Liver Physiol. 2009; 296:G572–G581.4. El-Badry AM, Graf R, Clavien PA. Omega 3 - Omega 6: What is right for the liver? J Hepatol. 2007; 47:718–725.5. Kikugawa K, Yasuhara Y, Ando K, Koyama K, Hiramoto K, Suzuki M. Protective effect of supplementation of fish oil with high n-3 polyunsaturated fatty acids against oxidative stress-induced DNA damage of rat liver in vivo. J Agric Food Chem. 2003; 51:6073–6079.6. Kirimlioglu V, Kirimlioglu H, Yilmaz S, Ozgor D, Coban S, Karadag N, et al. Effect of fish oil, olive oil, and vitamin E on liver pathology, cell proliferation, and antioxidant defense system in rats subjected to partial hepatectomy. Transplant Proc. 2006; 38:564–567.7. Marsman HA, de Graaf W, Heger M, van Golen RF, Ten Kate FJ, Bennink R, et al. Hepatic regeneration and functional recovery following partial liver resection in an experimental model of hepatic steatosis treated with omega-3 fatty acids. Br J Surg. 2013; 100:674–683.8. Silva RM, Malafaia O, Torres OJ, Czeczko NG, Marinho Junior CH, Kozlowski RK. Evaluation of liver regeneration diet supplemented with omega-3 fatty acids: experimental study in rats. Rev Col Bras Cir. 2015; 42:393–397.9. Drabkin DL, Austin JH. Spectrophotometric studies. II. Preparations from washed blood cells: nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem. 1935; 112:51–55.10. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.11. Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem. 1990; 190:360–365.12. Nebot C, Moutet M, Huet P, Xu JZ, Yadan JC, Chaudiere J. Spectrophotometric assay of superoxide dismutase activity based on the activated autoxidation of a tetracyclic catechol. Anal Biochem. 1993; 214:442–451.13. Deisseroth A, Dounce AL. Catalase: physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970; 50:319–375.14. Gerard-Monnier D, Erdelmeier I, Regnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998; 11:1176–1183.15. Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982; 710:197–211.16. Dragin N, Smani M, Arnaud-Dabernat S, Dubost C, Moranvillier I, Costet P, et al. Acute oxidative stress is associated with cell proliferation in the mouse liver. FEBS Lett. 2006; 580:3845–3852.17. Ozsoy M, Gonul Y, Bal A, Ozkececi ZT, Celep RB, Adali F, et al. Effect of IL-18 binding protein on hepatic ischemia-reperfusion injury induced by infrarenal aortic occlusion. Ann Surg Treat Res. 2015; 88:92–99.18. Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000; 408:239–247.19. Dong-Yun S, Yu-Ru D, Shan-Lin L, Ya-Dong Z, Lian W. Redox stress regulates cell proliferation and apoptosis of human hepatoma through Akt protein phosphorylation. FEBS Lett. 2003; 542:60–64.20. Garcia JJ, Lopez-Pingarron L, Almeida-Souza P, Tres A, Escudero P, García-Gil FA, et al. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: a review. J Pineal Res. 2014; 56:225–237.21. Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: part II. Redox biology in pathologic hepatocytes and implications for intervention. J Surg Res. 2011; 167:96–112.22. Yoon SY, Kim CY, Han HJ, Lee KO, Song TJ. Protective effect of ischemic postconditioning against hepatic ischemic reperfusion injury in rat liver. Ann Surg Treat Res. 2015; 88:241–245.23. Carnovale CE, Scapini C, Alvarez ML, Favre C, Monti J, Carrillo MC. Nitric oxide release and enhancement of lipid peroxidation in regenerating rat liver. J Hepatol. 2000; 32:798–804.24. Nandivada P, Cowan E, Carlson SJ, Chang M, Gura KM, Puder M. Mechanisms for the effects of fish oil lipid emulsions in the management of parenteral nutrition-associated liver disease. Prostaglandins Leukot Essent Fatty Acids. 2013; 89:153–158.25. Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int Arch Allergy Immunol. 2003; 132:310–321.26. Melo JU, Santos JM, Kimura Ode S, Campos Junior MM, Melo RB, Vasconcelos PR. Effects of fatty acids on liver regeneration in rats. Rev Col Bras Cir. 2010; 37:351–357.27. Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T. Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition. 2011; 27:334–337.28. Cai W, Wu J, Hong L, Xu Y, Tang Q, Shi C. Oxidative injury and hepatocyte apoptosis in total parenteral nutrition-associated liver dysfunction. J Pediatr Surg. 2006; 41:1663–1668.29. Capussotti L, Muratore A, Baracchi F, Lelong B, Ferrero A, Regge D, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg. 2008; 143:978–982.30. McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006; 16:R551–R560.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor: Effects of Omega-3 Fatty Acids on Erectile Dysfunction in a Rat Model of Atherosclerosis-induced Chronic Pelvic Ischemia
- Omega-3 and Menopause
- Omega-3 Index as a Risk Factor for Cardiovascular Disease and Its Application to Korean Population
- Manganese-induced Oxidative Stress in the Corpus Striatum of the Rat Brain
- Omega-3 Polyunsaturated Fatty Acid for Cholestasis due to Bile Duct Paucity