Clin Exp Vaccine Res.
2013 Jan;2(1):4-7.
Vaccines today, vaccines tomorrow: a perspective
- Affiliations
-
- 1International Vaccine Institute, Seoul, Korea. cloucq@ivi.int
Abstract
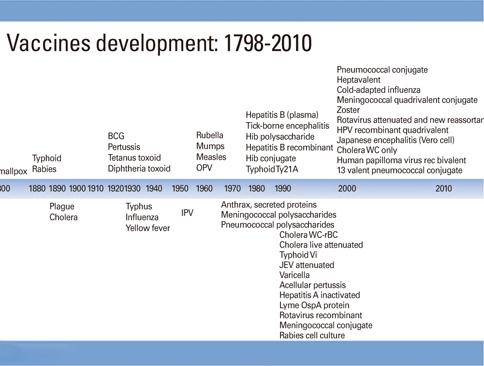
- Vaccines are considered as one of the major contributions of the 20th century and one of the most cost effective public health interventions. The International Vaccine Institute has as a mission to discover, develop and deliver new and improved vaccines against infectious diseases that affects developing nations. If Louis Pasteur is known across the globe, vaccinologists like Maurice Hilleman, Jonas Salk and Charles Merieux are known among experts only despite their contribution to global health. Thanks to a vaccine, smallpox has been eradicated, polio has nearly disappeared, Haemophilus influenzae B, measles and more recently meningitis A are controlled in many countries. While a malaria vaccine is undergoing phase 3, International Vaccine Institute, in collaboration with an Indian manufacturer has brought an oral inactivated cholera vaccine to pre-qualification. The field of vaccinology has undergone major changes thanks to philanthropists such as Bill and Melinda Gates, initiatives like the Decade of Vaccines and public private partnerships. Current researches on vaccines have more challenging targets like the dengue viruses, malaria, human immunodeficiency virus, the respiratory syncytial virus and nosocomial diseases. Exciting research is taking place on new adjuvants, nanoparticles, virus like particles and new route of administration. An overcrowded infant immunization program, anti-vaccine groups, immunizing a growing number of elderlies and delivering vaccines to difficult places are among challenges faced by vaccinologists and global health experts.
Keyword
MeSH Terms
-
Cholera
Communicable Diseases
Cooperative Behavior
Dengue Virus
Developing Countries
Haemophilus influenzae
HIV
Humans
Hypogonadism
Immunization Programs
Infant
Malaria
Measles
Meningitis
Missions and Missionaries
Mitochondrial Diseases
Nanoparticles
Ophthalmoplegia
Poliomyelitis
Public Health
Public-Private Sector Partnerships
Respiratory Syncytial Viruses
Smallpox Vaccine
Vaccines
Viruses
Hypogonadism
Mitochondrial Diseases
Ophthalmoplegia
Smallpox Vaccine
Vaccines
Figure
Reference
-
1. International Vaccine Institute [Internet]. c2012. cited 2012 Nov 1. Seoul: Internationl Vaccine Institute;Available from: http://www.ivi.int.2. Lombard M, Pastoret PP, Moulin AM. A brief history of vaccines and vaccination. Rev Sci Tech. 2007. 26:29–48.
Article3. GAVI Alliance. "Miracle of vaccines" will help deliver on Africa development [Internet]. c2012. cited 2012 Nov 2. Washington, DC: GAVI Alliance;Available from: http://www.gavialliance.org/library/news/gavi-features/2012/miracle-of-vaccines-deliver-on-africa-development/.4. Polio Global Eradication Initiative [Internet]. cited 2012 Nov 2. Geneva: Polio Global Eradication Initiative;Available from: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx.5. Cowgill KD, Ndiritu M, Nyiro J, et al. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006. 296:671–678.
Article6. Laforce M. Introduction and impact of a new group A meningococcal conjugate vaccine in Africa. Global Vaccines 202X: Access, Equity, Ethics. 2011 May 2-4; Philadelphia.7. Sur D, Lopez AL, Kanungo S, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009. 374:1694–1702.
Article8. Agnandji ST, Lell B, Soulanoudjingar SS, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011. 365:1863–1875.
Article9. The RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012. 367:2284–2295.10. The Global Vaccine Safety Initiative (GVSI) [Internet]. c2012. cited 2012 Nov 1. Geneva: World Health Organization;Available from: http://www.who.int/vaccine_safety/initiative/en/.11. Decade of Vaccines Collaboration. Action plan [Internet]. c2012. cited 2012 Nov 1. Barcelona: Decade of Vaccines Collaboration;Available from: http://www.dovcollaboration.org/action-plan/.12. Bill and Melinda Gates Foundation. Global health [Internet]. c2010. cited 2012 Nov 1. Seattle: Bill and Melinda Gates Foundation;Available from: http://www.gatesfoundation.org/global-health/Documents/global-health-strategy-overview.pdf.13. Coller BA, Clements DE. Dengue vaccines: progress and challenges. Curr Opin Immunol. 2011. 23:391–398.
Article14. MVI PATH. Malaria vaccine approaches three types [Internet]. c2012. cited 2012 Nov 1. Washington, DC: MVI PATH;Available from: http://www.malariavaccine.org/malvac-approaches.php#transmission.15. Gerhard W, Mozdzanowska K, Zharikova D. Prospects for universal influenza virus vaccine. Emerg Infect Dis. 2006. 12:569–574.
Article16. Gerding DN. Treatment and prevention options for Clostridium difficile infection. 2012. Bethesda: National Foundation for Infectious Diseases.17. Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol. 2009. 16:1709–1719.
Article18. Czerkinsky C. Sublingual immunization as a strategy for inducing broad based immune responses in mucosal and extra-mucosal tissues [Internet]. cited 2012 Nov 1. Seoul: International Vaccine Institute;Available from: http://www.who.int/vaccine_research/diseases/influenza/Cecil_Czerkinsky_3rdBroadspectrum.pdf.19. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998. 351:637–641.
Article20. Report of the ad-hoc consultation on ageing and immunization [Internet]. c2011. cited 2012 Nov 1. Geneva: World Health Organization;http://whqlibdoc.who.int/hq/2011/WHO_IVB_11.10_eng.pdf.