J Rheum Dis.
2011 Jun;18(2):85-93.
Inhibition of the IL-1beta-induced Expression of Matrix Metalloproteinases by Controlled Release of IL-1 Receptor Antagonist Using Injectable and Thermo-reversible Gels in Human Osteoarthritis Chondrocytes
- Affiliations
-
- 1Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea.
- 2Department of Bioengineering, College of Engineering, Hanyang University, Seoul, Korea. yongheekim@hanyang.ac.kr
- 3Institute of Rheumatism, Hanyang University, Seoul, Korea.
- 4Department of Surgery for Rheumatism, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea.
Abstract
OBJECTIVE
IL-1beta is involved in the degradation of articular cartilage in various arthritides, including osteoarthritis (OA). Competitive inhibition of IL-1beta by IL-1 receptor antagonists (IL-1Ra) may represent a pathogenesis-based strategy for inhibiting degradation of the cartilage matrix. We investigated the hypothesis that controlled release of IL-1Ra using injectable, thermoreversible and complex coacervate combination gels as drug delivery systems might reduce matrix degradation in OA.
METHODS
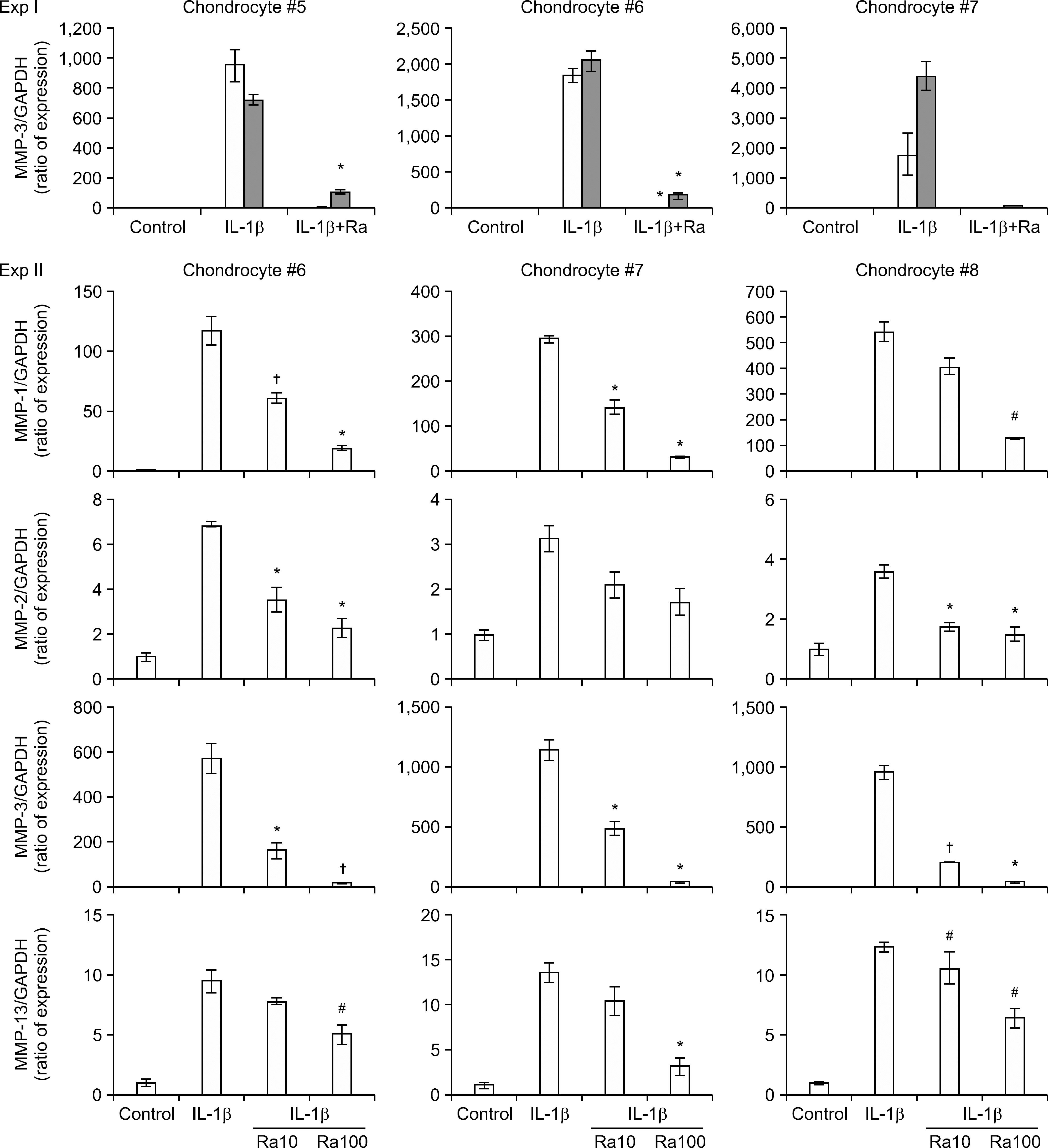
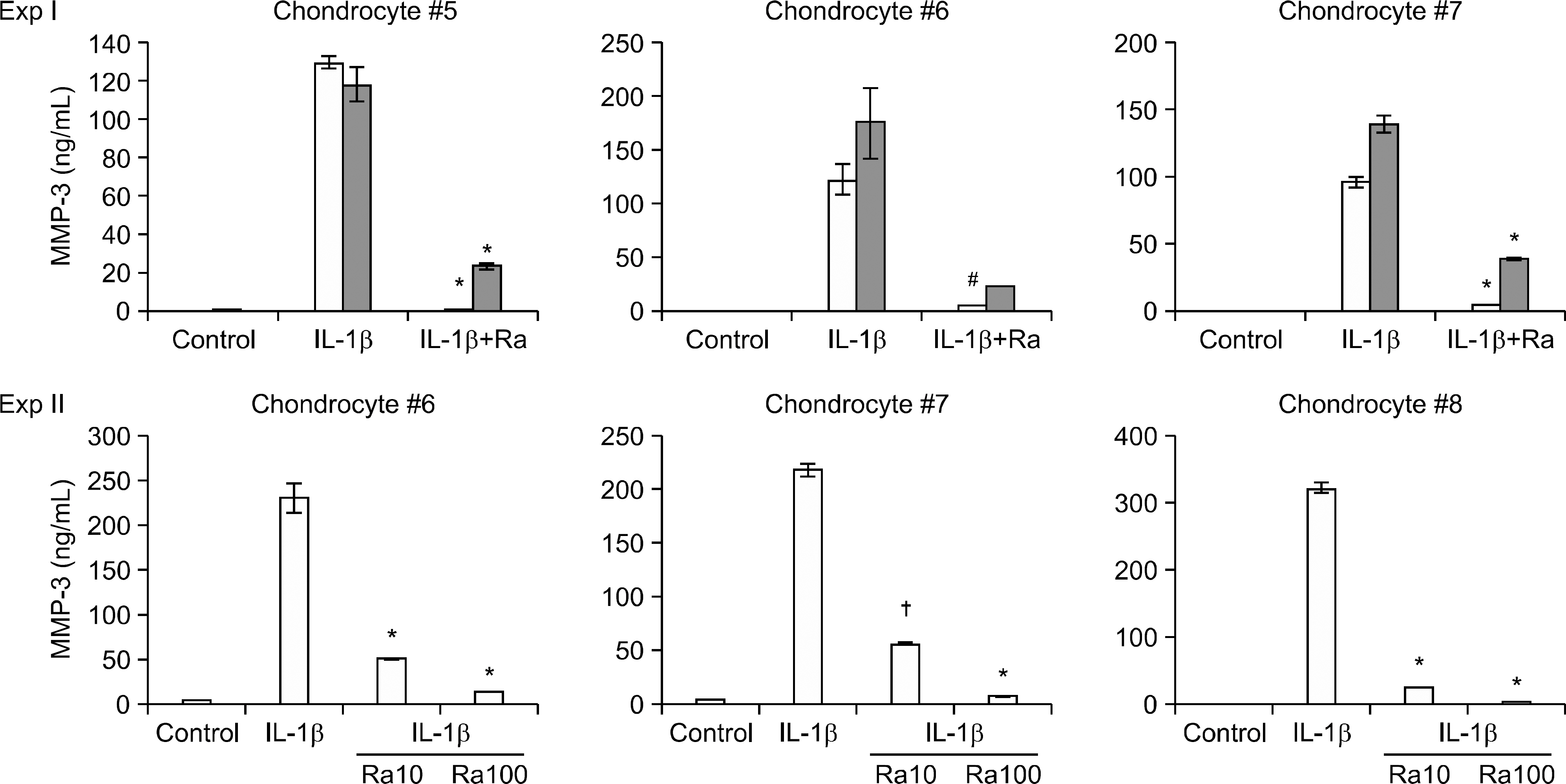
Thermoreversible combination gels that can be injected into joints were formed in aqueous solution by making a complex coacervate with recombinant human IL-1Ra (anakinra) and cationic macromolecules, and this was followed by co-formulation with methylcellulose as a negative thermosensitive polysaccharide. Gels containing anakinra were positioned in the upper insert of a transwell system and human OA chondrocytes were placed in the lower compartment and then they were stimulated with IL-1beta. The expression of matrix metalloproteinases (MMPs) was examined by performing real time PCR and ELISA.
RESULTS
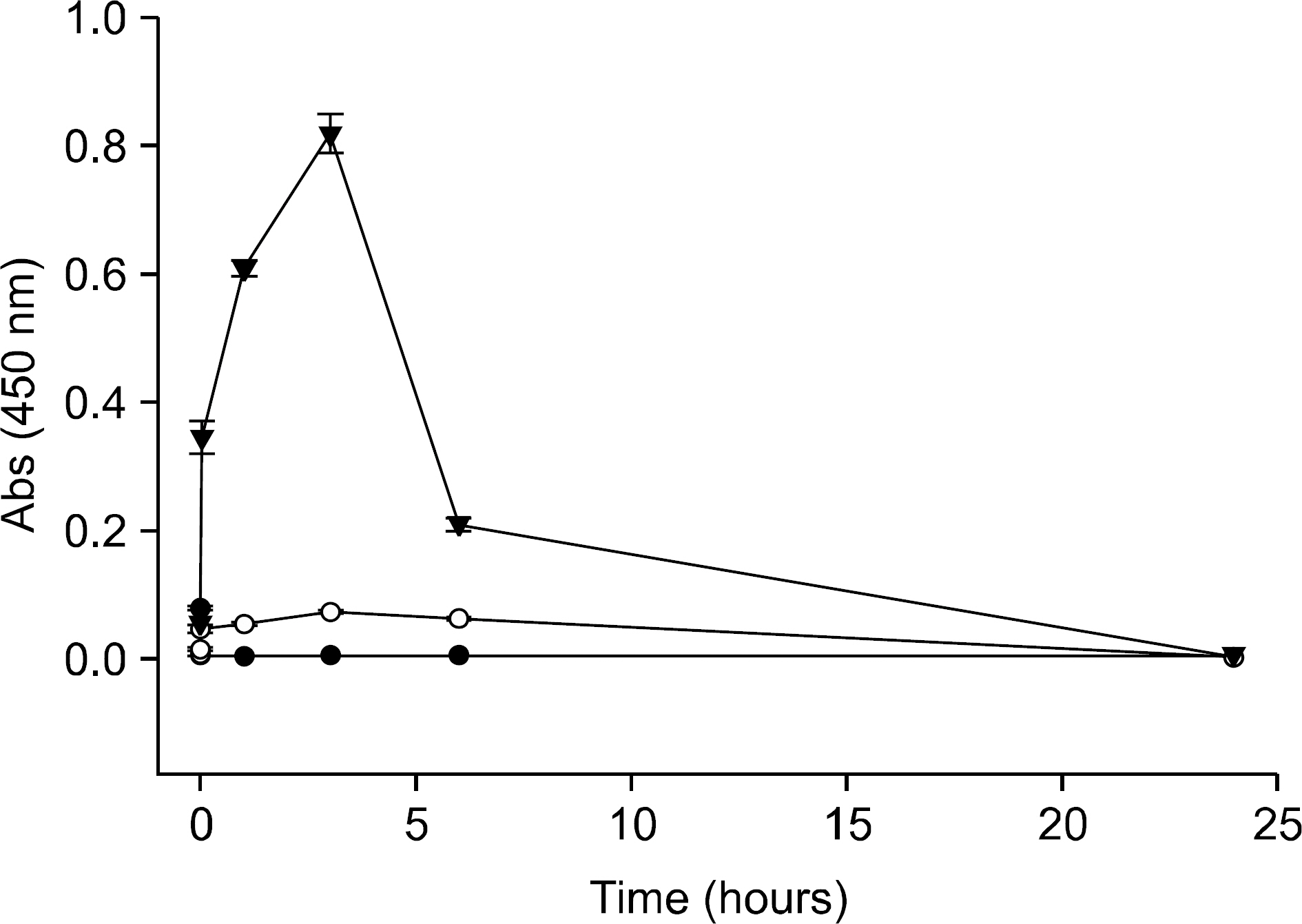
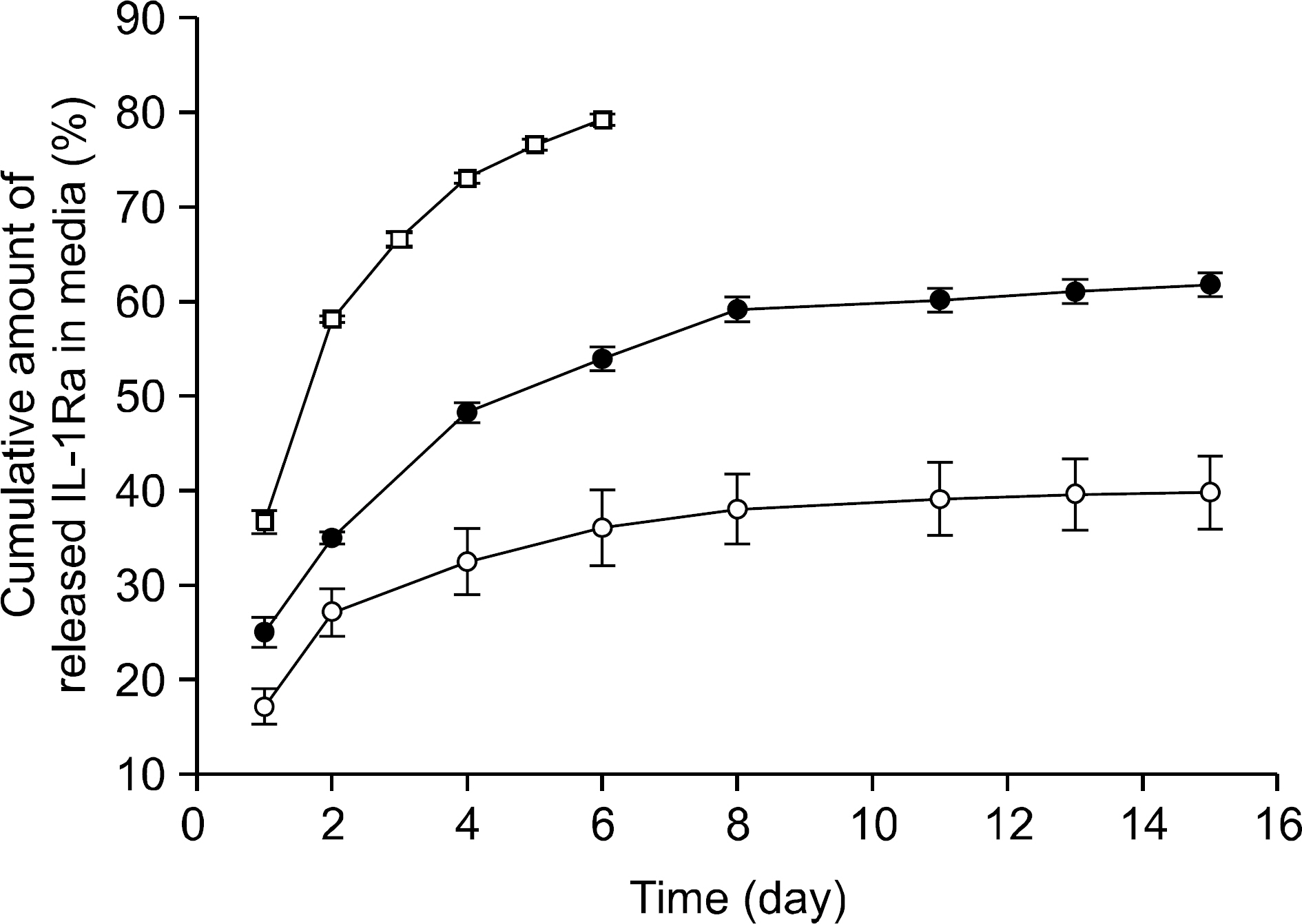
Complex coacervation between anakinra and protamine was successfully completed. IL-1Ra was released from the gels in a sustained release pattern for extended periods with minimal initial bursts. IL-1beta markedly enhanced the expression of MMP. The IL-1Ra released from the gels significantly inhibited the IL-1beta-induced MMP expression in the chondrocytes.
CONCLUSION
We developed and optimized a novel injectable and thermoreversible gel system for the controlled release of IL-1Ra, and this drug delivery system effectively inhibited the IL-1beta-induced MMP expression of chondrocytes in a transwell system. Intra-articular local delivery of injectable and thermoreversible gels containing IL-1Ra into knees has the potential to provide prolonged therapy based on the pathophysiology of knee OA.
Keyword
MeSH Terms
-
Arthritis
Cartilage
Cartilage, Articular
Chondrocytes
Drug Delivery Systems
Gels
Humans
Interleukin 1 Receptor Antagonist Protein
Interleukin-1
Joints
Knee
Matrix Metalloproteinases
Methylcellulose
Osteoarthritis
Real-Time Polymerase Chain Reaction
Gels
Interleukin 1 Receptor Antagonist Protein
Interleukin-1
Matrix Metalloproteinases
Methylcellulose
Figure
Reference
-
References
1. Issa SN, Sharma L. Epidemiology of osteoarthritis: an update. Curr Rheumatol Rep. 2006; 8:7–15.
Article2. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000; 43:1905–15.3. Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collage-nase-1 expression. Arthritis Rheum. 1996; 39:1535–44.
Article4. Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997; 40:1012–9.
Article5. Fernandes J, Tardif G, Martel-Pelletier J, Lascau-Coman V, Dupuis M, Moldovan F, et al. In vivo transfer of in-terleukin-1 receptor antagonist gene in osteoarthritic rab-bit knee joints: prevention of osteoarthritis progression. Am J Pathol. 1999; 154:1159–69.6. Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J Orthop Res. 2004; 22:742–50.
Article7. Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, et al. Dual transduction of in-sulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005; 23:118–26.8. Wang HJ, Yu CL, Kishi H, Motoki K, Mao ZB, Muraguchi A. Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin Med J (Engl). 2006; 119:1365–73.
Article9. Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005; 32:1317–23.10. Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004; (427 Suppl):S27–36.
Article11. Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and in-terferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994; 54:85–99.12. Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteogly-can synthesis by interleukin-1. Biochem Biophys Res Commun. 1994; 200:142–8.13. Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002; 13:323–40.
Article14. Slack J, McMahan CJ, Waugh S, Schooley K, Spriggs MK, Sims JE, et al. Independent binding of interleukin-1 alpha and interleukin-1 beta to type I and type II interleukin-1 receptors. J Biol Chem. 1993; 268:2513–24.
Article15. Iqbal I, Fleischmann R. Treatment of osteoarthritis with anakinra. Curr Rheumatol Rep. 2007; 9:31–5.
Article16. Xia J, Dubin PL. Protein-polyelectrolyte complexes. Dubin PL, Bock J, Davis R, Schulz DN, Thies C, editors. Macromolecular Complexes in Chemistry and Biology. p. 247–71. Berlin: Springer-Verlag Telos;1994.
Article17. Poznansky MJ, Juliano RL. Biological approaches to the controlled delivery of drugs: a critical review. Pharmacol Rev. 1984; 36:277–336.18. Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995; 6:332–51.
Article19. Jin KM, Kim YH. Injectable, thermoreversible and complex coacervate combination gels for protein drug delivery. J Control Release. 2008; 127:249–56.
Article20. Bau B, Gebhard PM, Haag J, Knorr T, Bartnik E, Aigner T. Relative messenger RNA expression profiling of collagenases and aggrecanases in human articular chondrocytes in vivo and in vitro. Arthritis Rheum. 2002; 46:2648–57.
Article21. Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005; 52:128–35.22. Tetlow LC, Adlam DJ, Woolley DE. Matrix metal-loproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001; 44:585–94.
Article23. Qvist P, Bay-Jensen AC, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008; 58:1–7.
Article24. Abramson SB, Yazici Y. Biologics in development for rheumatoid arthritis: relevance to osteoarthritis. Adv Drug Deliv Rev. 2006; 58:212–25.
Article25. Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 2006; 20:879–96.
Article26. Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009; 61:344–52.
Article27. Vignon E, Balblanc JC, Mathieu P, Louisot P, Richard M. Metalloprotease activity, phospholipase A2 activity and cytokine concentration in osteoarthritis synovial fluids. Osteoarthritis Cartilage. 1993; 1:115–20.
Article28. Chang DM, Chang SY, Yeh MK, Lai JH. The pharma-cokinetics of interleukin-1 receptor antagonist in Chinese subjects with rheumatoid arthritis. Pharmacol Res. 2004; 50:371–6.
Article29. Richette P, Francois M, Vicaut E, Fitting C, Bardin T, Corvol M, et al. A high interleukin 1 receptor antagonist/IL-1beta ratio occurs naturally in knee osteoarthritis. J Rheumatol. 2008; 35:1650–4.30. Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, et al. Aggrecan degradation in human articular cartilage explants is mediated by both ADA-MTS-4 and ADAMTS-5. Arthritis Rheum. 2007; 56:575–85.
Article31. Lin Z, Fitzgerald JB, Xu J, Willers C, Wood D, Grodzinsky AJ, et al. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J Orthop Res. 2008; 26:1230–7.
Article32. Martel-Pelletier J, Pelletier JP. Osteoarthritis: A single injection of anakinra for treating knee OA? Nat Rev Rheumatol. 2009; 5:363–4.33. Church LD, McDermott MF. Canakinumab, a fully-human mAb against IL-1beta for the potential treatment of inflammatory disorders. Curr Opin Mol Ther. 2009; 11:81–9.34. Burns CM, Wortmann RL. Gout therapeutics: new drugs for an old disease. Lancet. 2011; 377:165–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of gangliosides from deer bone extract on the gene expressions of matrix metalloproteinases and collagen type II in interleukin-1β-induced osteoarthritic chondrocytes
- miR-139 modulates MCPIP1/IL-6 expression and induces apoptosis in human OA chondrocytes
- Effects of ageing and arthritic disease on nitric oxide production by human articular chondrocytes
- Compound K Inhibits Interleukin-1β-induced Expression of Inflammatory Mediators and Matrix Metalloproteinases by Inhibiting Mitogen-activated Protein Kinase Activation in Chondrocytes
- Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway