J Gastric Cancer.
2015 Dec;15(4):270-277. 10.5230/jgc.2015.15.4.270.
Beginner Surgeon's Initial Experience with Distal Subtotal Gastrectomy for Gastric Cancer Using a Minimally Invasive Approach
- Affiliations
-
- 1Department of Surgery, CHA Bundang Medical Center, CHA University, Seongnam, Korea. aug-79@cha.ac.kr
- KMID: 2391562
- DOI: http://doi.org/10.5230/jgc.2015.15.4.270
Abstract
- PURPOSE
Minimally invasive gastrectomy (MIG), including laparoscopic distal subtotal gastrectomy (LDG) and robotic distal subtotal gastrectomy (RDG), is performed for gastric cancer, and requires a learning period. However, there are few reports regarding MIG by a beginner surgeon trained in MIG for gastric cancer during surgical residency and fellowship. The aim of this study was to report our initial experience with MIG, LDG, and RDG by a trained beginner surgeon.
MATERIALS AND METHODS
Between January 2014 and February 2015, a total of 36 patients (20 LDGs and 16 RDGs) underwent MIG by a beginner surgeon during the learning period, and 13 underwent open distal subtotal gastrectomy (ODG) by an experienced surgeon in Bundang CHA Medical Center. Demographic characteristics, operative findings, and short-term outcomes were evaluated for the groups.
RESULTS
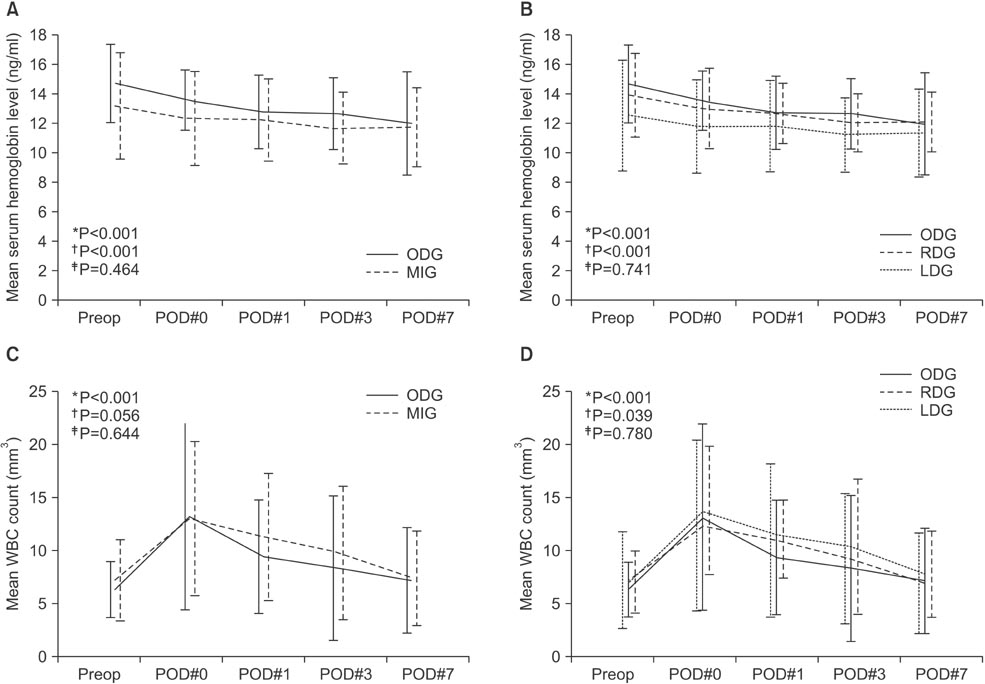
MIG was safely performed without open conversion in all patients and there was no mortality in either group. There was no significant difference between the groups in demographic factors except for body mass index. There were significant differences in extent of lymph node dissection (LND) (D2 LND: ODG 8.3% vs. MIG 55.6%, P=0.004) and mean operative time (ODG 178.8 minutes vs. MIG 254.7 minutes, P<0.001). The serial changes in postoperative hemoglobin level (P=0.464) and white blood cell count (P=0.644) did not show significant differences between the groups. There were no significant differences in morbidity.
CONCLUSIONS
This study showed that the operative and short-term outcomes of MIG for gastric cancer by a trained beginner surgeon were comparable with those of ODG performed by an experienced surgeon.
Keyword
MeSH Terms
Figure
Reference
-
1. Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N. Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007; 245:68–72.2. Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010; 251:417–420.3. Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014; 32:627–633.4. Park do J, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS)Group. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012; 26:1548–1553.5. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14:113–123.6. Okines A, Verheij M, Allum W, Cunningham D, Cervantes A. ESMO Guidelines Working Group. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21:Suppl 5. v50–v54.7. Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, et al. National Comprehensive Cancer Network. Gastric cancer, version 22013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013; 11:531–546.8. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014; 14:87–104.9. Kim HI, Park MS, Song KJ, Woo Y, Hyung WJ. Rapid and safe learning of robotic gastrectomy for gastric cancer: multidimensional analysis in a comparison with laparoscopic gastrectomy. Eur J Surg Oncol. 2014; 40:1346–1354.10. Stolzenburg JU, Rabenalt R, Do M, Horn LC, Liatsikos EN. Modular training for residents with no prior experience with open pelvic surgery in endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2006; 49:491–498.11. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.12. Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc. 2009; 23:1204–1211.13. Woo Y, Hyung WJ, Pak KH, Inaba K, Obama K, Choi SH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg. 2011; 146:1086–1092.14. Hyung WJ, Lim JS, Song J, Choi SH, Noh SH. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg. 2008; 207:e6–e11.15. Hyung WJ, Song C, Cheong JH, Choi SH, Noh SH. Factors influencing operation time of laparoscopy-assisted distal subtotal gastrectomy: analysis of consecutive 100 initial cases. Eur J Surg Oncol. 2007; 33:314–319.16. Woo Y, Hyung WJ, Kim HI, Obama K, Son T, Noh SH. Minimizing hepatic trauma with a novel liver retraction method: a simple liver suspension using gauze suture. Surg Endosc. 2011; 25:3939–3945.17. Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. 2002; 195:284–287.18. Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopyassisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005; 11:7508–7511.19. Park SS, Kim MC, Park MS, Hyung WJ. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc. 2012; 26:60–67.20. Yang SJ, Ahn EJ, Park SH, Kim JH, Park JM. The early experience of laparoscopy-assisted gastrectomy for gastric cancer at a low-volume center. J Gastric Cancer. 2010; 10:241–246.21. Kim MG, Kwon SJ. Comparison of the outcomes for laparoscopic gastrectomy performed by the same surgeon between a low-volume hospital and a high-volume center. Surg Endosc. 2014; 28:1563–1570.22. Ding J, Liao GQ, Liu HL, Liu S, Tang J. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol. 2012; 105:297–303.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter to the Editor Concerning "Short-Term Outcomes of Laparoscopic Total Gastrectomy Performed by a Single Surgeon Experienced in Open Gastrectomy: Review of Initial Experience"
- Laparoscopic Distal Gastrectomy for Gastric Cancer
- The Influence of Operative Approach on Food Retention after Open and Laparoscopy-Assisted Distal Gastrectomy (LADG) for Gastric Cancer
- Recent advances in minimally invasive surgery for gastric cancer
- Is Laparoscopic Approach Also Safe for the Treatment of Remnant Gastric Cancer?