Transl Clin Pharmacol.
2017 Sep;25(3):153-156. 10.12793/tcp.2017.25.3.153.
Comparison of pharmacokinetic characteristics of sildenafil citrate chewable tablets and film-coated tablets in healthy male subjects
- Affiliations
-
- 1Seoul National University College of Medicine and Hospital, Seoul 03080, Republic of Korea. leejh413@snu.ac.kr
- 2Korea United Pharm. INC., Seoul 06116, Republic of Korea.
- 3Caleb Multilab INC., Seoul 06745, Republic of Korea.
- 4Industrial Pharmaceutial Sciences, School of Pharmacy, Sungkyunkwan University, Seoul 16149, Republic of Korea.
- 5Chungnam National University College of Medicine and Hospital, Daejeon 35015, Republic of Korea. boniii@cnu.ac.kr
- KMID: 2390936
- DOI: http://doi.org/10.12793/tcp.2017.25.3.153
Abstract
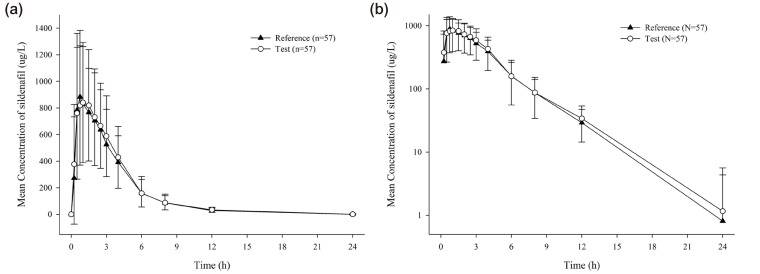
- UI14SDF100CW is a chewable tablet of sildenafil citrate, which was developed to improve compliance through convenience of administration. The purpose of this study was to compare the pharmacokinetic (PK) properties of sildenafil citrate chewable tablets (UI14SDF100CW) and conventional sildenafil citrate film-coated tablets (Viagra®, Pfizer). A randomized, open-label, single dose, two-treatment, two-period, two-way crossover study was conducted in 60 healthy male volunteers. In each period, the subjects received a single oral dose of UI14SDF100CW or Viagra® (both tablets contain 140.45 mg of sildenafil citrate, which is equivalent to 100 mg of sildenafil). Serial blood samples were collected up to 24 h post-dose for PK analysis. The plasma concentration of sildenafil was determined using a validated HPLC-MS/MS assay. PK parameters of sildenafil were calculated using non-compartmental methods. The plasma concentration-time profiles of sildenafil in both formulations were similar. For UI14SDF100CW, the C(max) and AUC(last) of sildenafil were 1068.69 ± 458.25 (mean ± standard deviation) mg/L and 3580.59 ± 1680.29 h·mg/L, and the corresponding values for Viagra® were 1146.84 ± 501.70 mg/L and 3406.35 ± 1452.31 h·/L, respectively. The geometric mean ratios (90% confidence intervals) of UI14SDF100CW to Viagra® for C(max) and AUC(last) were 0.933 (0.853-1.021) and 1.034 (0.969-1.108), respectively, which met the bioequivalence criteria of Korean regulatory agency. In conclusion, UI14SDF100CW and Viagra® showed similar PK properties. Therefore, UI14SDF100CW can be an alternative to sildenafil for the treatment of erectile dysfunction, providing better compliance.
Keyword
MeSH Terms
Figure
Reference
-
1. Yang DH, Jeong JY, Jang SN, Lee SK, Choi YJ, Kim DH. Prevalence and Risk Factors for Erectile Dysfunction in Aging Men: Hallym Aging Study (HAS). Korean J Urol. 2007; 48:1258–1276.
Article2. Nehra A, Kulaksizoglu H. Global Perspectives and Controversies in the Epidemiology of Male Erectile Dysfunction. Curr Opin Urol. 2002; 12:493–496. PMID: 12409879.
Article3. Boolell M, Allesn MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP177 specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996; 8:47–52. PMID: 8858389.4. Moreland RB, Goldstein I, Traish A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998; 62:PL 309–PL 318.
Article5. Eardley I, Ellis P, Boolell M, Wulff M. Onset and duration of action of sildenafil citrate for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2002; 53(Suppl 1):61S–65S. PMID: 11879261.6. Gupta M, Kovar A, Meibohm B. The Clinical Pharmacokinetics 186 of Phosphodiesterase-5 Inhibitorsfor Erectile Dysfunction. J Clin Pharmacol. 2005; 45:987–1003. PMID: 16100293.7. Lewis JR, Johnson DR. A novel method for the Determination of sildenafil (Viagra) and its metabolite (UK-103, 320) in postmortem specimen using LC-MS/MS and LC-MS/MS.MS, A final reprt by U.S Department fo Transport Federal Aviation administration. 2000. 5. Accessed Aug 10 2017. https://www.faa.gov/data_research/med_humanfacs/oamtechreports/2000s/media/00_20.pdf.8. Kanjanawart S, Kongyingyoes B, Gaysornsiri D, Tangsucharit P, Puapairoj P, Vannaprasaht S, et al. Bioequivalence and Pharmacokinetic Study of Sildenafil in Healthy Thai Male Volunteers. Srinagarind Med J. 2008; 23:38–44.9. Valenzuela F, Davila G, Ibañez Y, Garcia L, Crownover P, Gómez-Palacio R, et al. Relative Bioavailability of Chewable and Conventional Film-Coated Tablet Formulations of Sildenafil 100mg in Healthy Volunteers Under Fasting Conditions. J Bioequiv Availab. 2011; 3:207–210.10. Stavros F, Kramer WG, Wilkins MR. The effects of sitaxentan on sildenafil pharmacokinetics and pharmacodynamics in healthy subjects. Br J Clin Pharmacol. 2010; 69:23–26. DOI: 10.1111/j.1365-2125.2009.03541.x. PMID: 20078609.
Article11. European Medicines Agency, ASSESSMENT REPORT FOR Sildenafil ratiopharm. 2009. Accessed Aug 10 2017. http://www.ema.europa.eu/docs/en_GB/document_library/PAR_-_Public_assessment_report/human/001080/WC500068025.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of a Visual Field Defect with Optical Coherence Tomography Changes after Sildenafil Citrate Overdose
- Retinal Hemorrhage Associated with Viagra (sildenafil citrate)
- Mirodenafil for the Treatment of Erectile Dysfunction: A Systematic Review of the Literature
- Swollowed Pills Resembling Urinary Stone
- A Case of Transient Color Anomaly and Persistent Visual Field Defect after Sildenafil Citrate Overdose