Anat Cell Biol.
2017 Sep;50(3):214-218. 10.5115/acb.2017.50.3.214.
Expression of glucose transporters in the developing rat skin
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. eunjulee@amc.seoul.kr
- KMID: 2390483
- DOI: http://doi.org/10.5115/acb.2017.50.3.214
Abstract
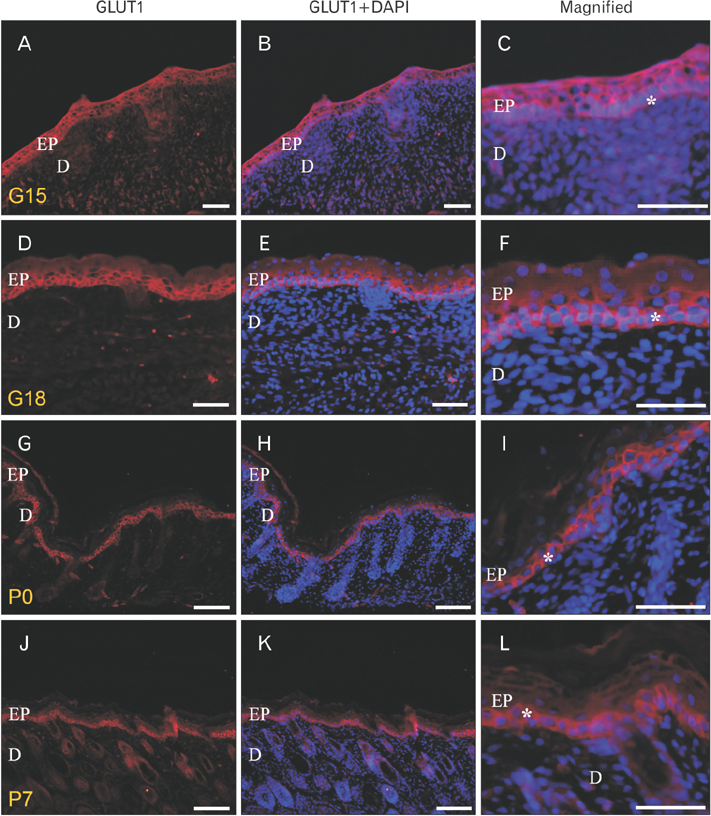
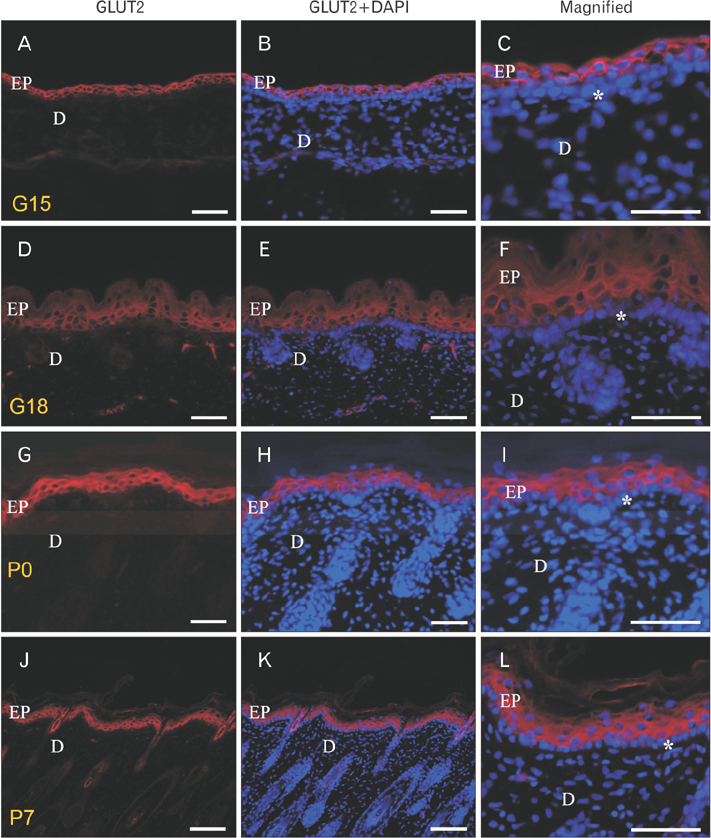
- We found the changed distribution of glucose transporter (GLUT) proteins in the skin during rat development. At 15 days of gestation, GLUT1 and 2 proteins were expressed in the stratum corneum of epidermal cells. In postnatal skin, however, GLUT1 and 2 exhibit different expression patterns. While GLUT1 expression becomes more restricted to the stratum basale with development, GLUT2 was found mainly in stratum spinosum and granulosum, but not being localized in the stratum basale at any stages of perinatal skin development. Considering all these, it can be speculated that each GLUT protein plays its specific role in different epidermal layers and that the glucose used in mammalian skin in utero could be originated from the amniotic fluid during skin development.
Keyword
MeSH Terms
Figure
Reference
-
1. Wilson-O'Brien AL, Patron N, Rogers S. Evolutionary ancestry and novel functions of the mammalian glucose transporter (GLUT) family. BMC Evol Biol. 2010; 10:152.2. Gould GW, Holman GD. The glucose transporter family: structure, function and tissue-specific expression. Biochem J. 1993; 295(Pt 2):329–341.3. Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996; 16:235–256.4. Shen S, Wertheimer E, Sampson SR, Tennenbaum T. Characterization of glucose transport system in keratinocytes: insulin and IGF-1 differentially affect specific transporters. J Invest Dermatol. 2000; 115:949–954.5. Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schürmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. Am J Physiol Endocrinol Metab. 2002; 282:E974–E976.6. Gherzi R, Melioli G, de Luca M, D'Agostino A, Distefano G, Guastella M, D'Anna F, Franzi AT, Cancedda R. “HepG2/erythroid/brain” type glucose transporter (GLUT1) is highly expressed in human epidermis: keratinocyte differentiation affects GLUT1 levels in reconstituted epidermis. J Cell Physiol. 1992; 150:463–474.7. Davies CA, Jeziorska M, Freemont AJ, Herrick AL. The differential expression of VEGF, VEGFR-2, and GLUT-1 proteins in disease subtypes of systemic sclerosis. Hum Pathol. 2006; 37:190–197.8. Kuroki S, Yokoo S, Terashi H, Hasegawa M, Komori T. Epithelialization in oral mucous wound healing in terms of energy metabolism. Kobe J Med Sci. 2009; 55:E5–E15.9. Tochio T, Tanaka H, Nakata S. Glucose transporter member 1 is involved in UVB-induced epidermal hyperplasia by enhancing proliferation in epidermal keratinocytes. Int J Dermatol. 2013; 52:300–308.10. Bell GI, Burant CF, Takeda J, Gould GW. Structure and function of mammalian facilitative sugar transporters. J Biol Chem. 1993; 268:19161–19164.11. Takata K. Glucose transporters in the transepithelial transport of glucose. J Electron Microsc (Tokyo). 1996; 45:275–284.12. Wertheimer E, Sasson S, Cerasi E, Ben-Neriah Y. The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins. Proc Natl Acad Sci U S A. 1991; 88:2525–2529.13. Giordana B, Milani A, Grimaldi A, Farneti R, Casartelli M, Ambrosecchio MR, Digilio MC, Leonardi MG, de Eguileor M, Pennacchio F. Absorption of sugars and amino acids by the epidermis of Aphidius ervi larvae. J Insect Physiol. 2003; 49:1115–1124.14. de Eguileor M, Grimaldi A, Tettamanti G, Valvassori R, Leonardi MG, Giordana B, Tremblay E, Digilio MC, Pennacchio F. Larval anatomy and structure of absorbing epithelia in the aphid parasitoid Aphidius ervi Haliday (Hymenoptera, Braconidae). Arthropod Struct Dev. 2001; 30:27–37.15. Shepard TH, Tanimura T, Park HW. Glucose absorption and utilization by rat embryos. Int J Dev Biol. 1997; 41:307–314.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Expression of GABA Transporters in Developing Rat Retina
- Differential Expression of System L Amino Acid Transporters in Wound Healing Process of Rat Skin
- Sympathetic and parasympathetic regulation of sodium transporters and water channels in rat submandibular gland
- High glucose, unsaturated and saturated fatty acids differentially regulate expression of ATP-binding cassette transporters ABCA1 and ABCG1 in human macrophages
- Altered expression of sodium transporters and water channels following sympathetic and parasympathetic denervation in rat submandibular gland