Anat Cell Biol.
2017 Sep;50(3):187-199. 10.5115/acb.2017.50.3.187.
Histological study on the effect of nicotine on adult male guinea pig thin skin
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine, Assiut University, Assiut, Egypt. sohair_eltony@yahoo.com
- KMID: 2390480
- DOI: http://doi.org/10.5115/acb.2017.50.3.187
Abstract
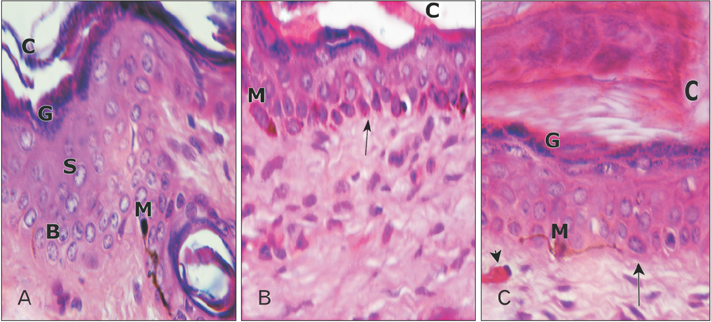
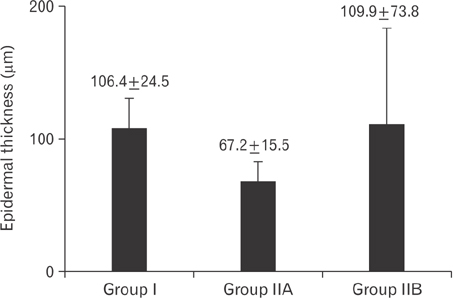
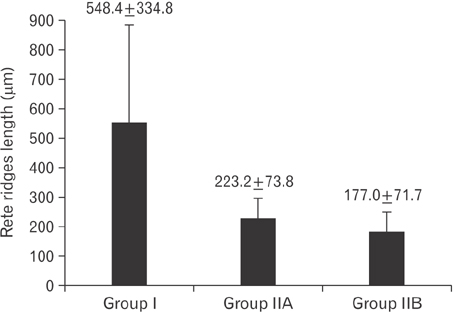
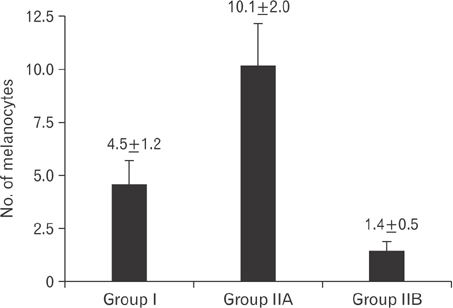
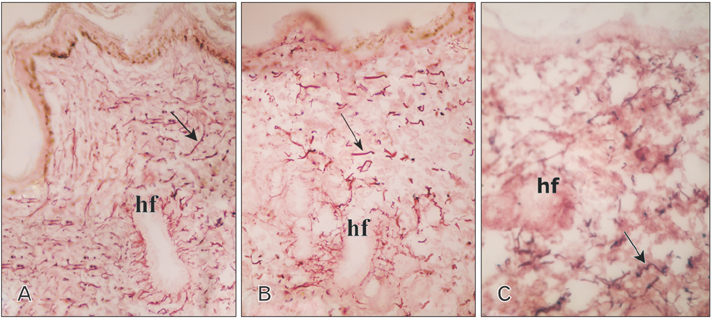
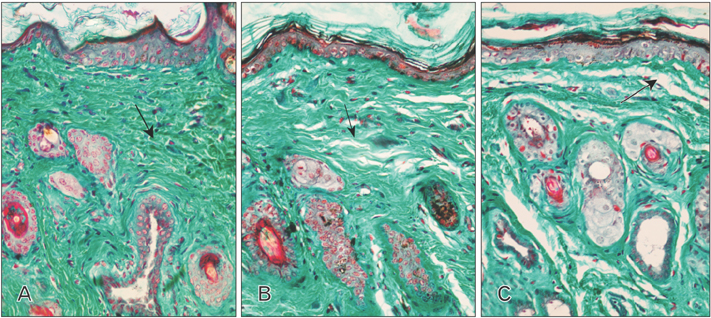
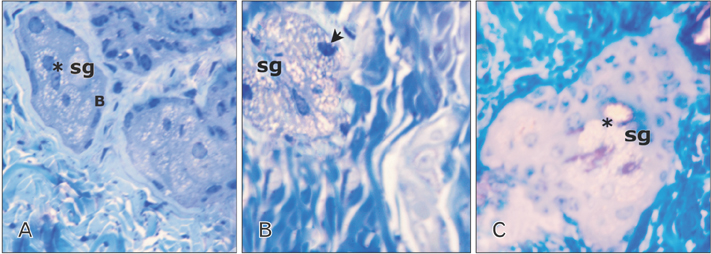
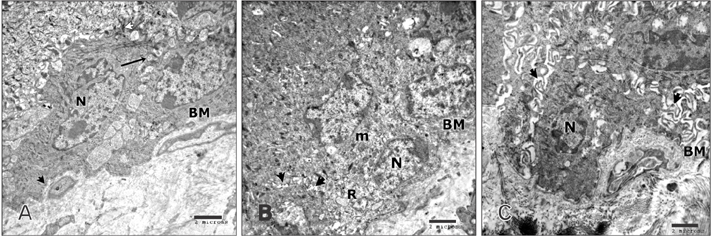
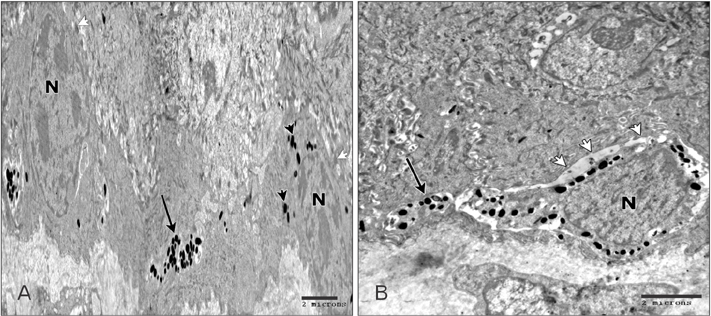
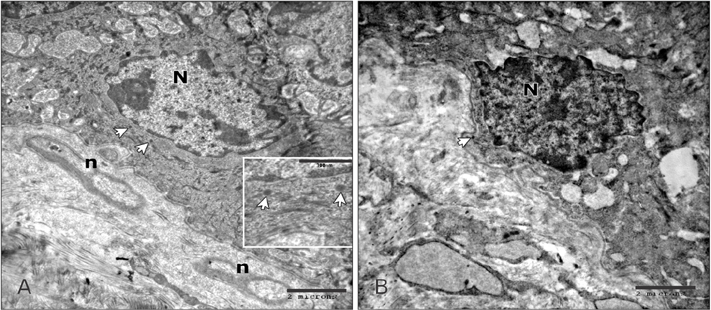
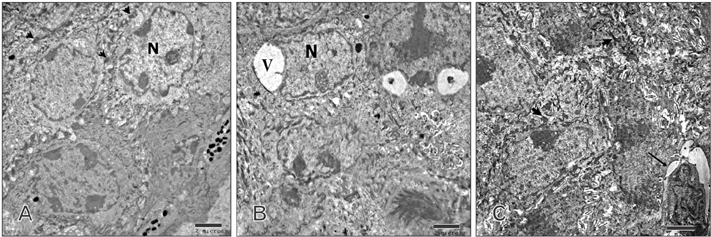
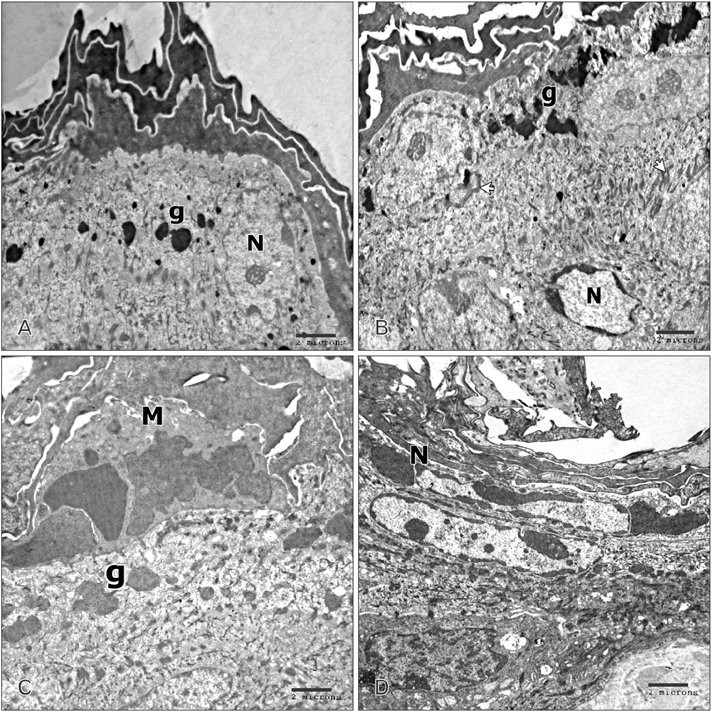
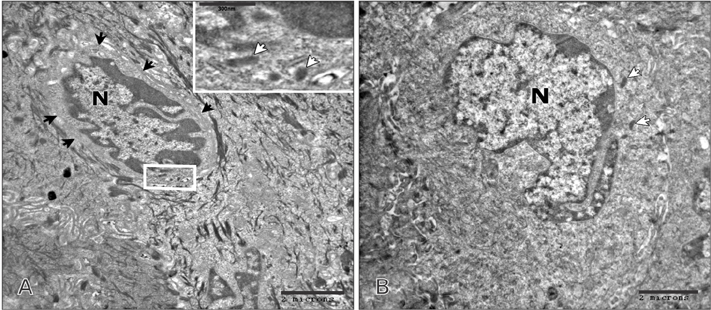
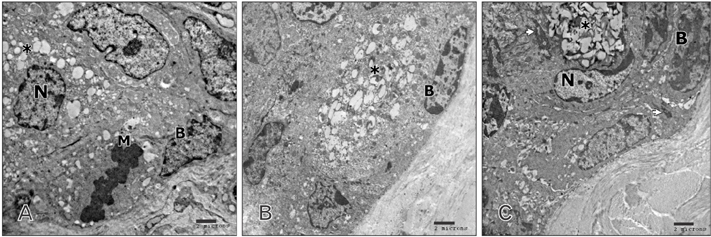
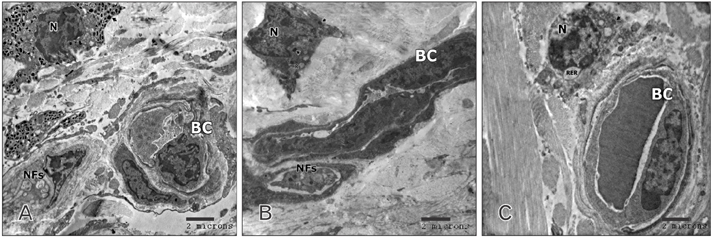
- Tobacco smoking has been identified as an important factor in premature skin aging to detect the histological changes occurred in adult male guinea pig thin skin under the influence of low and high doses of nicotine; which constitutes approximately 0.6%-3.0% of the dry weight of tobacco. Fifteen adult male pigmented guinea pigs were equally divided into three groups: group I, control; group IIA, low dose nicotine treated; 3 mg/kg subcutaneously for 4 weeks; and group IIB, high dose nicotine treated; 6 mg/kg subcutaneously for 4 weeks. Specimens from the back thin skin were processed for light and electron microscopy. Nicotine administration revealed flattened dermo-epidermal junction and reduced rete ridges formation. Collagen bundles were disorganized with increased spaces between them. A reduction in the amount of elastic fibers in the dermis were also observed compared to group I. Ultrastructurally, keratinocytes had hyperchromatic nuclei, intracytoplasmic vacuoles, disruption of desmosomal junctions, irregular tonofilaments distribution, and increased inter-cellular spaces. These changes were more pronounced with high dose nicotine administration. The epidermal thickness was reduced in low dose nicotine administration. But, high dose nicotine administration revealed increased epidermal thickness compared to the control group. Nicotine induced structural changes of adult male guinea pig thin skin. These changes were more pronounced with high dose nicotine administration.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegmund B, Leitner E, Pfannhauser W. Determination of the nicotine content of various edible nightshades (Solanaceae) and their products and estimation of the associated dietary nicotine intake. J Agric Food Chem. 1999; 47:3113–3120.2. Mayer B. How much nicotine kills a human? Tracing back the generally accepted lethal dose to dubious self-experiments in the nineteenth century. Arch Toxicol. 2014; 88:5–7.3. Caponnetto P, Campagna D, Papale G, Russo C, Polosa R. The emerging phenomenon of electronic cigarettes. Expert Rev Respir Med. 2012; 6:63–74.4. Misery L. Nicotine effects on skin: are they positive or negative? Exp Dermatol. 2004; 13:665–670.5. Tortora GJ, Grabowski SR. Principles of anatomy and physiology. New York: Harper Collins College Publishers;1993.6. Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ. Cigarette smoking: risk factor for premature facial wrinkling. Ann Intern Med. 1991; 114:840–844.7. Yin L, Morita A, Tsuji T. Skin aging induced by ultraviolet exposure and tobacco smoking: evidence from epidemiological and molecular studies. Photodermatol Photoimmunol Photomed. 2001; 17:178–183.8. Koh JS, Kang H, Choi SW, Kim HO. Cigarette smoking associated with premature facial wrinkling: image analysis of facial skin replicas. Int J Dermatol. 2002; 41:21–27.9. Fore J. A review of skin and the effects of aging on skin structure and function. Ostomy Wound Manage. 2006; 52:24–35.10. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008; 30:87–95.11. Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007; 190:269–319.12. Abdel-Hafez AM, Elgayar SA, Husain OA, Thabet HS. Effect of nicotine on the structure of cochlea of guinea pigs. Anat Cell Biol. 2014; 47:162–170.13. Drury RA, Wallington EA. Carelton's histology technique. 5th ed. Oxford: Oxford University Press;1980.14. Gupta PD. Ultrastructural study on semithin section. Sci Tools. 1983; 30:6–7.15. Inan S, Oztukcan S, Vatansever S, Ermertcan AT, Zeybek D, Oksal A, Giray G, Muftuoglu S. Histopathological and ultrastructural effects of glycolic acid on rat skin. Acta Histochem. 2006; 108:37–47.16. Barbero AM, Frasch HF. Pig and guinea pig skin as surrogates for human in vitro penetration studies: a quantitative review. Toxicol In Vitro. 2009; 23:1–13.17. Frasch HF, Barbero AM. A paired comparison between human skin and hairless guinea pig skin in vitro permeability and lag time measurements for 6 industrial chemicals. Cutan Ocul Toxicol. 2009; 28:107–113.18. Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979; 73:59–66.19. Baumann L. Skin ageing and its treatment. J Pathol. 2007; 211:241–251.20. Branchet MC, Boisnic S, Frances C, Robert AM. Skin thickness changes in normal aging skin. Gerontology. 1990; 36:28–35.21. Oriá RB, Ferreira FV, Santana EN, Fernandes MR, Brito GA. Study of age-related changes in human skin using histomorphometric and autofluorescence approaches. An Bras Dermatol. 2003; 78:425–434.22. Ozguner F, Koyu A, Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005; 21:21–26.23. Deruy E, Gosselin K, Vercamer C, Martien S, Bouali F, Slomianny C, Bertout J, Bernard D, Pourtier A, Abbadie C. MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS One. 2010; 5:e12712.24. Barr J, Sharma CS, Sarkar S, Wise K, Dong L, Periyakaruppan A, Ramesh GT. Nicotine induces oxidative stress and activates nuclear transcription factor kappa B in rat mesencephalic cells. Mol Cell Biochem. 2007; 297:93–99.25. Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002; 138:1462–1470.26. Fortes C, Mastroeni S, Leffondre K, Sampogna F, Melchi F, Mazzotti E, Pasquini P, Abeni D. Relationship between smoking and the clinical severity of psoriasis. Arch Dermatol. 2005; 141:1580–1584.27. Setty AR, Curhan G, Choi HK. Smoking and the risk of psoriasis in women: Nurses' health study II. Am J Med. 2007; 120:953–959.28. Li W, Han J, Qureshi AA. Smoking and risk of incident psoriatic arthritis in US women. Ann Rheum Dis. 2012; 71:804–808.29. Soroka Y, Ma’or Z, Leshem Y, Verochovsky L, Neuman R, Brégégère FM, Milner Y. Aged keratinocyte phenotyping: morphology, biochemical markers and effects of Dead Sea minerals. Exp Gerontol. 2008; 43:947–957.30. Wallstrom M, Sand L, Nilsson F, Hirsch JM. The long-term effect of nicotine on the oral mucosa. Addiction. 1999; 94:417–423.31. Hanioka T, Tanaka K, Ojima M, Yuuki K. Association of melanin pigmentation in the gingiva of children with parents who smoke. Pediatrics. 2005; 116:e186–e190.32. Claffey DJ, Stout PR, Ruth JA. 3H-nicotine, 3H-flunitrazepam, and 3H-cocaine incorporation into melanin: a model for the examination of drug-melanin interactions. J Anal Toxicol. 2001; 25:607–611.33. Tadakamadla J, Kumar S, Nagori A, Tibdewal H, Duraiswamy P, Kulkarni S. Effect of smoking on oral pigmentation and its relationship with periodontal status. Dent Res J (Isfahan). 2012; 9:S112–S114.34. Nakamura M, Ueda Y, Hayashi M, Kato H, Furuhashi T, Morita A. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013; 22:556–558.35. Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008; 1778:572–587.36. Xin S, Ye L, Man G, Lv C, Elias PM, Man MQ. Heavy cigarette smokers in a Chinese population display a compromised permeability barrier. Biomed Res Int. 2016; 2016:9704598.37. Wulf HC, Sandby-Møller J, Kobayasi T, Gniadecki R. Skin aging and natural photoprotection. Micron. 2004; 35:185–191.38. Ortolan MC, Biondo-Simões MD, Valenga Baroni ED, Auersvald A, Auersvald LA, Netto MR, Biondo-Simões R. Influence of aging on the skin quality of white-skinned women: the role of collagen, elastic material density, and vascularization. Rev Bras Cir Plast. 2013; 28:41–48.39. Bosset S, Bonnet-Duquennoy M, Barré P, Chalon A, Lazou K, Kurfurst R, Bonté F, Schnébert S, Disant F, Le Varlet B, Nicolas JF. Decreased expression of keratinocyte beta1 integrins in chronically sun-exposed skin in vivo. Br J Dermatol. 2003; 148:770–778.40. Ortonne JP, Marks R. Photodamaged skin: clinical signs, causes and management. London: Martin Dunitz Ltd.;1999.41. Imokawa G, Nakajima H, Ishida K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: over-expression of neprilysin plays an essential role. Int J Mol Sci. 2015; 16:7776–7795.42. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002; 11:398–405.43. Merker K, Sitte N, Grune T. Hydrogen peroxide-mediated protein oxidation in young and old human MRC-5 fibroblasts. Arch Biochem Biophys. 2000; 375:50–54.44. Gray H, Williams PL, Bannister LH. Gray's anatomy: the anatomical basis of medicine and surgery. 38th ed. New York: Churchill Livingstone;1995.45. Zouboulis CC, Eady A, Philpott M, Goldsmith LA, Orfanos C, Cunliffe WC, Rosenfield R. What is the pathogenesis of acne? Exp Dermatol. 2005; 14:143–152.46. Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008; 17:542–551.47. Kindt F, Wiegand S, Niemeier V, Kupfer J, Löser C, Nilles M, Kurzen H, Kummer W, Gieler U, Haberberger RV. Reduced expression of nicotinic alpha subunits 3, 7, 9 and 10 in lesional and nonlesional atopic dermatitis skin but enhanced expression of alpha subunits 3 and 5 in mast cells. Br J Dermatol. 2008; 159:847–857.48. Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, Razani-Boroujerdi S, Sopori ML. Nicotine inhibits Fc epsilon RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through alpha 7/alpha 9/alpha 10-nicotinic receptors. J Immunol. 2010; 185:588–596.49. Mishra NC, Rir-Sima-Ah J, Langley RJ, Singh SP, Peña-Philippides JC, Koga T, Razani-Boroujerdi S, Hutt J, Campen M, Kim KC, Tesfaigzi Y, Sopori ML. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol. 2008; 180:7655–7663.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exophthalmos Inhibitory Effect of Anti-EPS Serum
- Effect of pH on Physical Properties of Guinea Pig Skin
- Effect of nicotine on the structure of cochlea of guinea pigs
- Histological Changes of the Skin of a Guinea Pig after Irradiation with the Erbium YAG Laser
- Familial Trichophyton mentagrophytes Infection Transmitted from Guinea Pig