Ann Rehabil Med.
2017 Apr;41(2):273-278. 10.5535/arm.2017.41.2.273.
Immediate Effect of a Single Session of Whole Body Vibration on Spasticity in Children With Cerebral Palsy
- Affiliations
-
- 1Department of Rehabilitation Medicine and Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Korea. MEDICUS@yuhs.ac
- KMID: 2389486
- DOI: http://doi.org/10.5535/arm.2017.41.2.273
Abstract
OBJECTIVE
To investigate the immediate effect of a single session of whole body vibration (WBV) on lower extremity spasticity in children with cerebral palsy (CP).
METHODS
Seventeen children with spastic CP were included. A single session of WBV was administered: 10-minute WBV, 1-minute rest, and 10-minute WBV. The effects of WBV were clinically assessed with the Modified Ashworth Scale (MAS) and Modified Tardieu Scale (MTS) before and immediately, 30 minutes, 1 hour, 2 hours, 3 hours, and 4 hours after WBV.
RESULTS
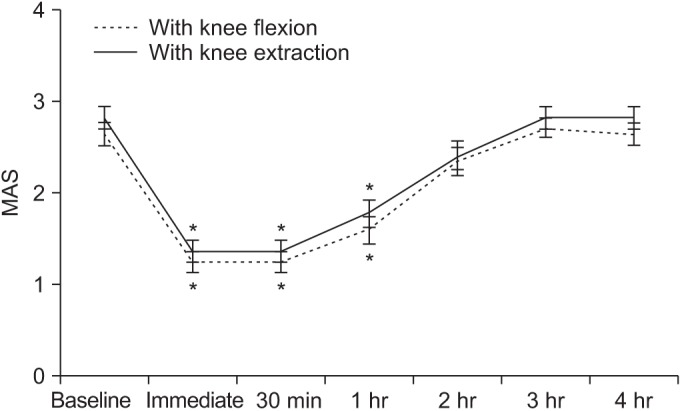
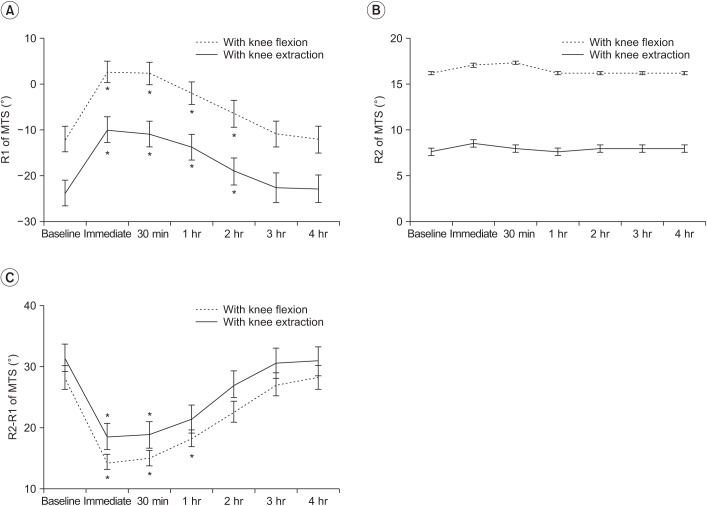
Spasticity of the ankle plantarflexor, as assessed by MAS and MTS scores, was reduced after WBV. Post-hoc analysis demonstrated that, compared to baseline, the MAS significantly improved for a period of 1 hour after WBV, and the R1 and R2-R1 of the MTS significantly improved for a period of 2 hours after WBV.
CONCLUSION
A single session of WBV improves spasticity of ankle plantarflexors for 1-2 hours in children with CP. Future studies are needed to test whether WBV is an effective preparation before physiotherapy and occupational therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004; 363:1619–1631. PMID: 15145637.
Article2. Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008; 50:334–340. PMID: 18355333.
Article3. Krigger KW. Cerebral palsy: an overview. Am Fam Physician. 2006; 73:91–100. PMID: 16417071.4. Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011; 32:75–99. PMID: 21165804.
Article5. Delecluse C, Roelants M, Verschueren S. Strength increase after whole-body vibration compared with resistance training. Med Sci Sports Exerc. 2003; 35:1033–1041. PMID: 12783053.
Article6. Slatkovska L, Alibhai SM, Beyene J, Cheung AM. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos Int. 2010; 21:1969–1980. PMID: 20407890.
Article7. Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007; 56:28–33. PMID: 17392595.
Article8. Cheng HY, Ju YY, Chen CL, Chuang LL, Cheng CH. Effects of whole body vibration on spasticity and lower extremity function in children with cerebral palsy. Hum Mov Sci. 2015; 39:65–72. PMID: 25461434.
Article9. Huang M, Liao LR, Pang MY. Effects of whole body vibration on muscle spasticity for people with central nervous system disorders: a systematic review. Clin Rehabil. 2017; 31:23–33. PMID: 26658333.
Article10. Katusic A, Alimovic S, Mejaski-Bosnjak V. The effect of vibration therapy on spasticity and motor function in children with cerebral palsy: a randomized controlled trial. NeuroRehabilitation. 2013; 32:1–8. PMID: 23422453.
Article11. Chan KS, Liu CW, Chen TW, Weng MC, Huang MH, Chen CH. Effects of a single session of whole body vibration on ankle plantarflexion spasticity and gait performance in patients with chronic stroke: a randomized controlled trial. Clin Rehabil. 2012; 26:1087–1095. PMID: 23035004.
Article12. Ness LL, Field-Fote EC. Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor Neurol Neurosci. 2009; 27:621–631. PMID: 20042786.
Article13. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987; 67:206–207. PMID: 3809245.
Article14. Boyd R, Barwood S, Baillieu C, Graham H. Validity of a clinical measure of spasticity in children with cerebral palsy in a double-blind randomized controlled clinical trial. Dev Med Child Neurol. 1998; 40(Suppl 78):7.15. Mackey AH, Walt SE, Lobb G, Stott NS. Intraobserver reliability of the modified Tardieu scale in the upper limb of children with hemiplegia. Dev Med Child Neurol. 2004; 46:267–272. PMID: 15077704.
Article16. Seo HG, Oh BM, Leigh JH, Chun C, Park C, Kim CH. Effect of Focal Muscle Vibration on Calf Muscle Spasticity: A Proof-of-Concept Study. PM R. 2016; 8:1083–1089. PMID: 26994885.
Article17. Yam WK, Leung MS. Interrater reliability of Modified Ashworth Scale and Modified Tardieu Scale in children with spastic cerebral palsy. J Child Neurol. 2006; 21:1031–1035. PMID: 17156693.
Article18. Kavounoudias A, Roll R, Roll JP. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci Lett. 1999; 266:181–184. PMID: 10465703.
Article19. Francis HP, Wade DT, Turner-Stokes L, Kingswell RS, Dott CS, Coxon EA. Does reducing spasticity translate into functional benefit? An exploratory meta-analysis. J Neurol Neurosurg Psychiatry. 2004; 75:1547–1551. PMID: 15489384.
Article20. Friedman BC, Goldman RD. Use of botulinum toxin A in management of children with cerebral palsy. Can Fam Physician. 2011; 57:1006–1073. PMID: 21918142.21. Levitt S. Treatment of cerebral palsy and motor delay. 5th ed. Hoboken: John Wiley & Sons;2010.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correction: Immediate Effect of a Single Session of Whole Body Vibration on Spasticity in Children With Cerebral Palsy
- Effect of Vibration Exercise Application on the Trunk Muscle Thickness in Children with Spastic Cerebral Palsy
- Percutaneous Selective Radiofrequency Thermocoagulation in the Treatment of Spastic Cerebral Palsy: A case report
- The Effects of Single Event Multi-level Chemoneurolysis on Upper Extremity Function in Children with Cerebral Palsy
- Effects of Botulinum Toxin A Treatment in Cerebral Palsy