Korean J Crit Care Med.
2017 May;32(2):174-181. 10.4266/kjccm.2016.00738.
Evaluation of Respiratory Dynamics in an Asymmetric Lung Compliance Model
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Jeju National University School of Medicine, Jeju, Korea.
- 2Department of Anesthesiology and Pain Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. yhlee0314@gmail.com
- KMID: 2384042
- DOI: http://doi.org/10.4266/kjccm.2016.00738
Abstract
- BACKGROUND
Unilateral lung hyperinflation develops in lungs with asymmetric compliance, which can lead to vital instability. The aim of this study was to investigate the respiratory dynamics and the effect of airway diameter on the distribution of tidal volume during mechanical ventilation in a lung model with asymmetric compliance.
METHODS
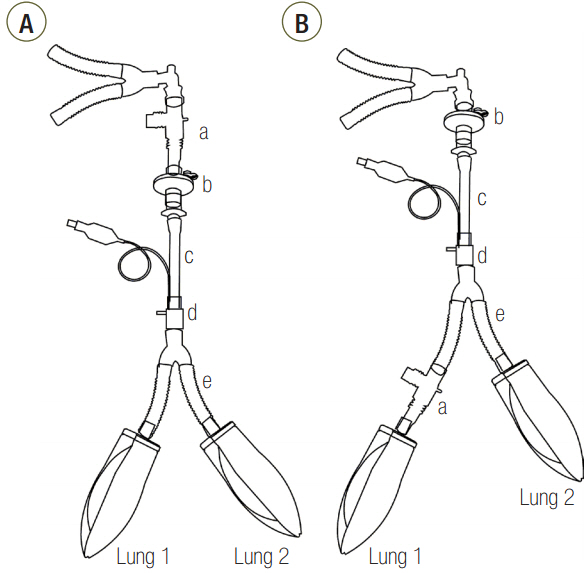
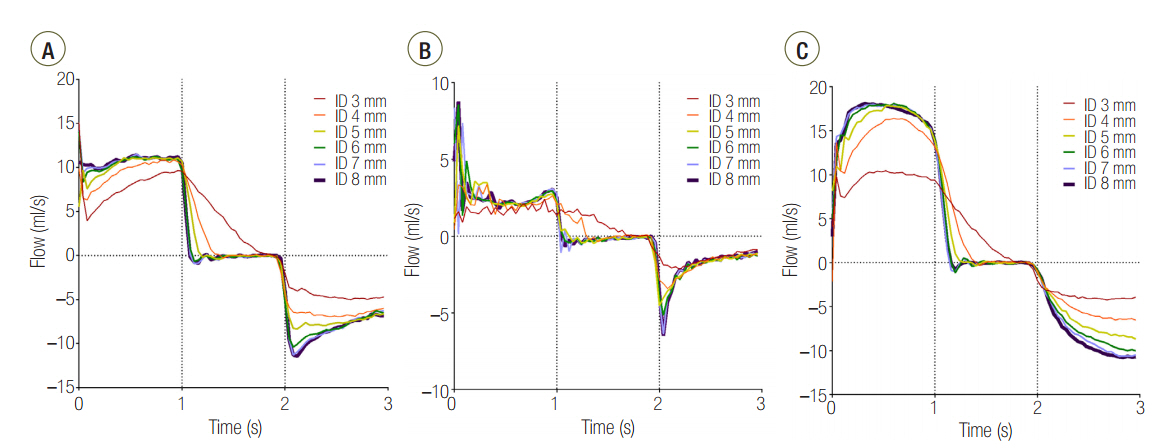
Three groups of lung models were designed to simulate lungs with a symmetric and asymmetric compliance. The lung model was composed of two test lungs, lung1 and lung2. The static compliance of lung1 in C15, C60, and C120 groups was manipulated to be 15, 60, and 120 ml/cmHâ‚‚O, respectively. Meanwhile, the static compliance of lung2 was fixed at 60 ml/cmHâ‚‚O. Respiratory variables were measured above (proximal measurement) and below (distal measurement) the model trachea. The lung model was mechanically ventilated, and the airway internal diameter (ID) was changed from 3 to 8 mm in 1-mm increments.
RESULTS
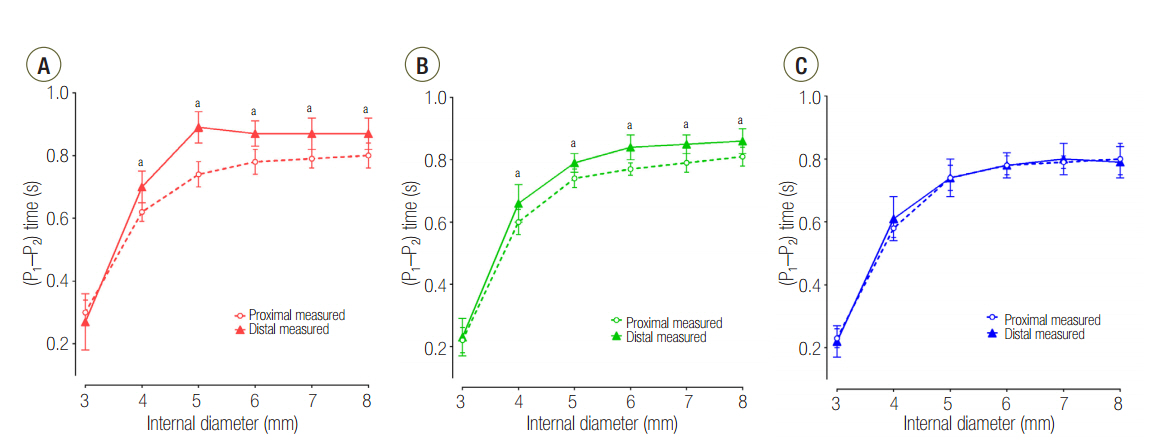
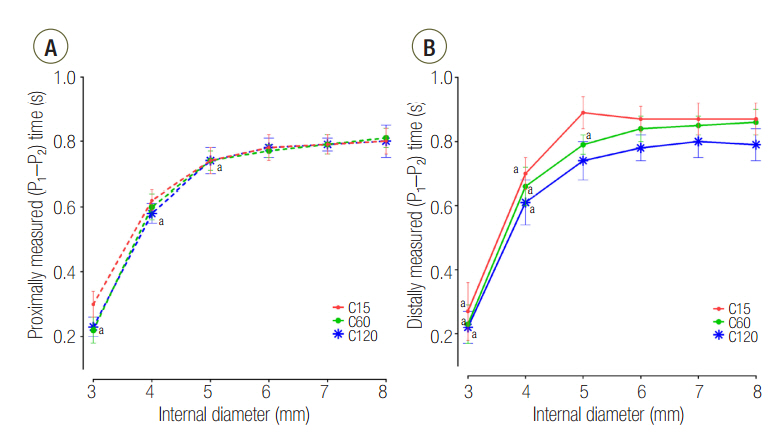
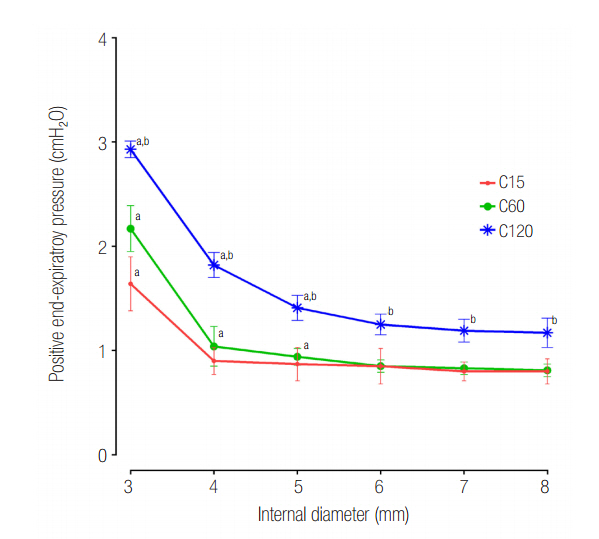
The mean ± standard deviation ratio of volumes distributed to each lung (VL1/VL2) in airway ID 3, 4, 5, 6, 7, and 8 were in order, 0.10 ± 0.05, 0.11 ± 0.03, 0.12 ± 0.02, 0.12 ± 0.02, 0.12 ± 0.02, and 0.12 ± 0.02 in the C15 group; 1.05 ± 0.16, 1.01 ± 0.09, 1.00 ± 0.07, 0.97 ± 0.09, 0.96 ± 0.06, and 0.97 ± 0.08 in the C60 group; and 1.46 ± 0.18, 3.06 ± 0.41, 3.72 ± 0.37, 3.78 ± 0.47, 3.77 ± 0.45, and 3.78 ± 0.60 in the C120 group. The positive end-expiratory pressure (PEEP) of lung1 was significantly increased at airway ID 3 mm (1.65 cmHâ‚‚O) in the C15 group; at ID 3, 4, and 5 mm (2.21, 1.06, and 0.95 cmHâ‚‚O) in the C60 group; and ID 3, 4, and 5 mm (2.92, 1.84, and 1.41 cmHâ‚‚O) in the C120 group, compared to ID 8 mm (P < 0.05).
CONCLUSIONS
In the C15 and C120 groups, the tidal volume was unevenly distributed to both lungs in a positive relationship with lung compliance. In the C120 group, the uneven distribution of tidal volume was improved when the airway ID was equal to or less than 4 mm, but a significant increase of PEEP was observed.
MeSH Terms
Figure
Cited by 1 articles
-
Is It Essential to Consider Respiratory Dynamics?
Youngjoon Kang
Korean J Crit Care Med. 2017;32(2):223-224. doi: 10.4266/kjccm.2017.00276.
Reference
-
References
1. Fitting JW. Respiratory muscles in chronic obstructive pulmonary disease. Swiss Med Wkly. 2001; 131:483–6.2. Ahmed SM, Athar M. Mechanical ventilation in patients with chronic obstructive pulmonary disease and bronchial asthma. Indian J Anaesth. 2015; 59:589–98.
Article3. Caramez MP, Borges JB, Tucci MR, Okamoto VN, Carvalho CR, Kacmarek RM, et al. Paradoxical responses to positive end-expiratory pressure in patients with airway obstruction during controlled ventilation. Crit Care Med. 2005; 33:1519–28.
Article4. Lumb AB. Nunn’s applied respiratory physiology. 7th ed. London: Churchill Livingstone;2010. 121–3.5. Venuta F, Boehler A, Rendina EA, De Giacomo T, Speich R, Schmid R, et al. Complications in the native lung after single lung transplantation. Eur J Cardiothorac Surg. 1999; 16:54–8.
Article6. Kollef MH, Turner JF. Intrinsic PEEP and unilateral llung hyperinflation: pathophysiology and clinical significance. Chest. 1992; 102:1220–4.7. Eveloff SE, Rounds S, Braman SS. Unilateral lung hyperinflation and herniation as a manifestation of intrinsic PEEP. Chest. 1990; 98:228–9.
Article8. Park SH, Yun SH, Park JC. Effects of upper airway obstruction on respiratory mechanics in a variable compliance model. Anesth Pain Med. 2011; 6:244–8.9. Al-Rawas N, Banner MJ, Euliano NR, Tams CG, Brown J, Martin AD, et al. Expiratory time constant for determinations of plateau pressure, respiratory system compliance, and total resistance. Crit Care. 2013; 17:R23.
Article10. Melo e Silva CA, Ventura CE. A simple model illustrating the respiratory system’s time constant concept. Adv Physiol Educ. 2006; 30:129–30.
Article11. Hess DR. Respiratory mechanics in mechanically ventilated patients. Respir Care. 2014; 59:1773–94.
Article12. Schumaker GL, Epstein SK. Managing acute respiratory failure during exacerbation of chronic obstructive pulmonary disease. Respir Care. 2004; 49:766–82.13. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164:770–7.
Article14. Puente-Maestu L, García de Pedro J, Martínez-Abad Y, Ruíz de Oña JM, Llorente D, Cubillo JM. Dyspnea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in COPD. Chest. 2005; 128:651–6.
Article15. Anglès R, Tenorio L, Roman A, Soler J, Rochera M, de Latorre FJ. Lung transplantation for emphysema. Lung hyperinflation: incidence and outcome. Transpl Int. 2005; 17:810–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is It Essential to Consider Respiratory Dynamics?
- Changes of Peak Inspiratory Airway Pressure and Compliance during One-Lung Ventilation Using Double Lumen Tube and Univent Tube
- Study on lung compliance in normal subjects patients with obstructive or restrictive lung diseases
- The effect on respiratory mechanics when using a Jackson surgical table in the prone position during spinal surgery
- Effects of upper airway obstruction on respiratory mechanics in a variable compliance model