Korean J Crit Care Med.
2017 May;32(2):106-123. 10.4266/kjccm.2017.00192.
The Role of Oliguria and the Absence of Fluid Administration and Balance Information in Illness Severity Scores
- Affiliations
-
- 1Department of Intensive Care, Austin Hospital, Melbourne, Australia. Rinaldo.BELLOMO@austin.org.au
- 2Australian and New Zealand Intensive Care Research Centre, School of Public Health and Preventive Medicine, Monash University, Prahran, Australia.
- 3School of Medicine, The University of Melbourne, Melbourne, Australia.
- KMID: 2384036
- DOI: http://doi.org/10.4266/kjccm.2017.00192
Abstract
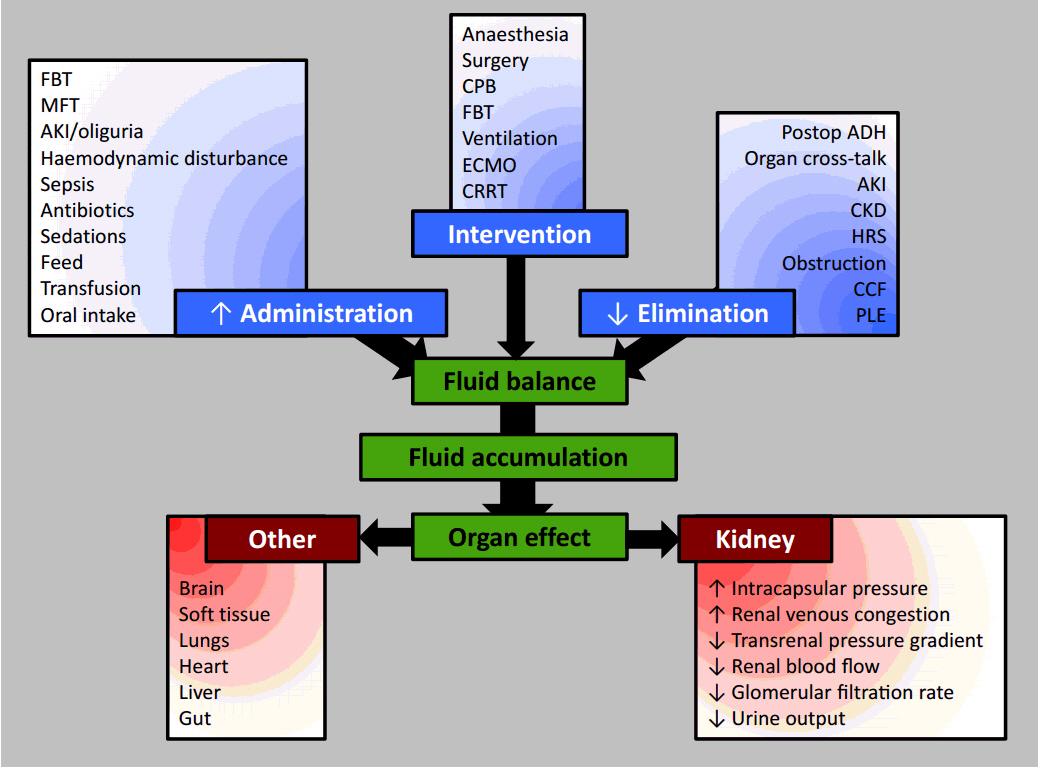
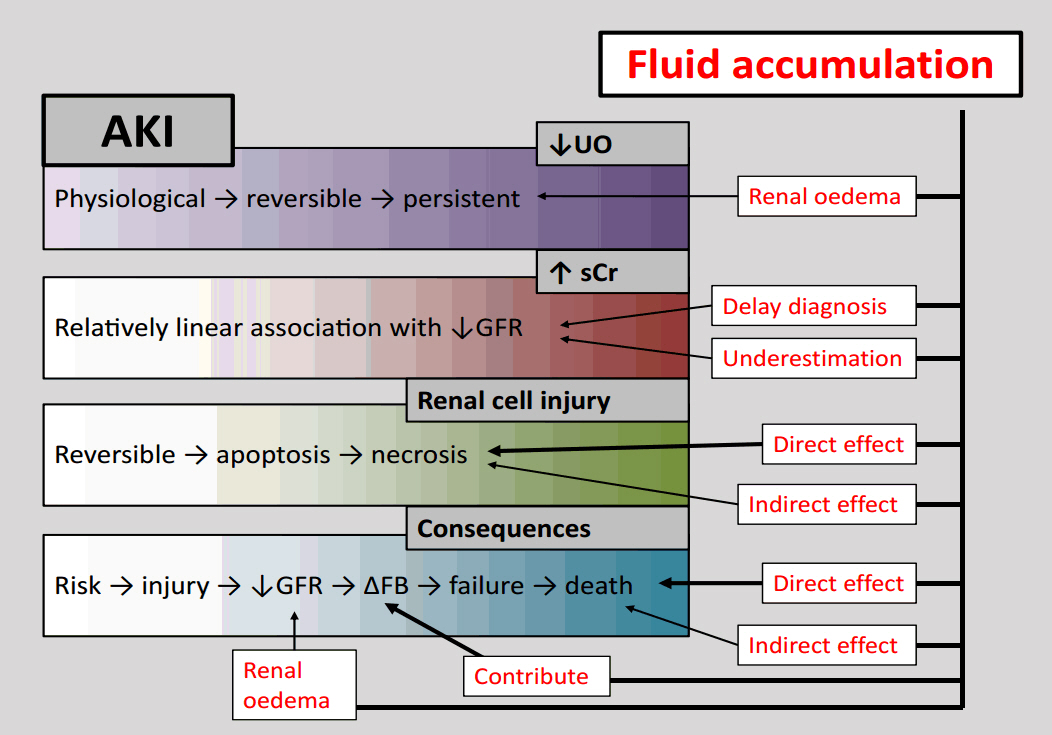
- Urinary examination has formed part of patient assessment since the earliest days of medicine. Current definitions of oliguria are essentially arbitrary, but duration and intensity of oliguria have been associated with an increased risk of mortality, and this risk is not completely attributable to the development of concomitant acute kidney injury (AKI) as defined by changes in serum creatinine concentration. The increased risk of death associated with the development of AKI itself may be modified by directly or indirectly by progressive fluid accumulation, due to reduced elimination and increased fluid administration. None of the currently extant major illness severity scoring systems or outcome prediction models use modern definitions of AKI or oliguria, or any values representative of fluid volumes variables. Even if a direct relationship with mortality is not observed, then it is possible that fluid balance or fluid volume variables mediate the relationship between illness severity and mortality in the renal and respiratory physiological domains. Fluid administration and fluid balance may then be an important, easily modifiable therapeutic target for future investigation. These relationships require exploration in large datasets before being prospectively validated in groups of critically ill patients from differing jurisdictions to improve prognostication and mortality prediction.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Eknoyan G. Origins of nephrology: hippocrates, the father of clinical nephrology. Am J Nephrol. 1988; 8:498–507.
Article2. Marketos SG, Eftychiadis AG, Diamandopoulos A. Acute renal failure according to ancient Greek and Byzantine medical writers. J R Soc Med. 1993; 86:290–3.3. Kouba E, Wallen EM, Pruthi RS. Uroscopy by Hippocrates and Theophilus: prognosis versus diagnosis. J Urol. 2007; 177:50–2.
Article4. Prowle JR, Liu YL, Licari E, Bagshaw SM, Egi M, Haase M, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care. 2011; 15:R172.
Article5. Chesley LC. The validity of the calculation of standard urea clearances from low urine volumes. J Clin Invest. 1937; 16:653–6.
Article6. Chesley LC. Renal excretion at low urine volumes and the mechanism of oliguria. J Clin Invest. 1938; 17:591–7.
Article7. Chesley LC. Urea excretion at low urine volumes: the calculation of “minimal” urea clearances. J Clin Invest. 1938; 17:119–23.
Article8. Chasis H, Smith HW. The excretion of urea in normal man and in subjects with glomerulonephritis. J Clin Invest. 1938; 17:347–58.9. Gamble JL. The Harvey lectures, series XLIII, 1946-1947: physiological information gained from studies on the life raft ration. Nutr Rev. 1989; 47:199–201.10. Creevy CD. Oliguria and anuria. Postgrad Med. 1954; 16:456–8.
Article11. Hopper JJ, Partridge JW. Anuria and oliguria: a review of symptoms, pathologic physiology and mortality rates. Calif Med. 1950; 72:415–21.12. Hopper JJ Jr, Partridge JW. Anuria and oliguria: treatment by conservative means, case report, with determination of blood volume and Na24 space. Calif Med. 1950; 73:42–53.13. Hay EB. Experiences with anuria and oliguria. AMA Arch Surg. 1951; 62:565–73.
Article14. Joekes AM. Discussion on anuria in medical conditions. Proc R Soc Med. 1957; 50:496–8.15. Brooks DK. The modern treatment of anuria and oliguria. Postgrad Med J. 1958; 34:583–7.
Article16. Milne MD. Discussion on anuria in medical conditions. Proc R Soc Med. 1957; 50:493–6.17. Hopkins RW, Sabga G, Bernardo P, Penn I, Simeone FA. Significance of post-traumatic and postoperative oliguria. Arch Surg. 1963; 87:320–30.
Article18. Harrington JT, Cohen JJ. Acute oliguria. N Engl J Med. 1975; 292:89–91.
Article19. Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998; 338:671–5.
Article20. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991; 100:1619–36.21. Schetz M, Hoste E. Understanding oliguria in the critically ill. Intensive Care Med. 2016; Sep. 12. [Epub].https://doi.org/10.1007/s00134-016-4537-7
Article22. Mathew OP, Jones AS, James E, Bland H, Groshong T. Neonatal renal failure: usefulness of diagnostic indices. Pediatrics. 1980; 65:57–60.
Article23. Gianantonio CA, Vitacco M, Mendilaharzu J, Mendilaharzu F, Rutty A. Acute renal failure in infancy and childhood: clinical course and treatment of 41 patients. J Pediatr. 1962; 61:660–78.24. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007; 71:1028–35.
Article25. Bezerra CT, Vaz Cunha LC, Libório AB. Defining reduced urine output in neonatal ICU: importance for mortality and acute kidney injury classification. Nephrol Dial Transplant. 2013; 28:901–9.
Article26. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure--definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8:R204–12.27. Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care. 2011; 1:13.
Article28. Md Ralib A, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Crit Care. 2013; 17:R112.
Article29. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009; 20:672–9.
Article30. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31.
Article31. Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013; 17:204.
Article32. Mandelbaum T, Lee J, Scott DJ, Mark RG, Malhotra A, Howell MD, et al. Empirical relationships among oliguria, creatinine, mortality, and renal replacement therapy in the critically ill. Intensive Care Med. 2013; 39:414–9.
Article33. Leedahl DD, Frazee EN, Schramm GE, Dierkhising RA, Bergstralh EJ, Chawla LS, et al. Derivation of urine output thresholds that identify a very high risk of AKI in patients with septic shock. Clin J Am Soc Nephrol. 2014; 9:1168–74.
Article34. Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012; 16:R197.
Article35. Vaara ST, Parviainen I, Pettila V, Nisula S, Inkinen O, Uusaro A, et al. Association of oliguria with the development of acute kidney injury in the critically ill. Kidney Int. 2015; Sep. 9. [Epub].https://doi.org/10.1038/ki.2015.26936. Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012; 81:819–25.
Article37. Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011; 7:209–17.
Article38. Barrantes F, Tian J, Vazquez R, Amoateng-Adjepong Y, Manthous CA. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008; 36:1397–403.
Article39. Morgan DJ, Ho KM. A comparison of nonoliguric and oliguric severe acute kidney injury according to the risk injury failure loss end-stage (RIFLE) criteria. Nephron Clin Pract. 2010; 115:c59–65.
Article40. Joannidis M, Metnitz B, Bauer P, Schusterschitz N, Moreno R, Druml W, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med. 2009; 35:1692–702.
Article41. Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011; 80:760–7.
Article42. Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011; 39:2659–64.
Article43. Han SS, Kang KJ, Kwon SJ, Wang SJ, Shin SH, Oh SW, et al. Additional role of urine output criterion in defining acute kidney injury. Nephrol Dial Transplant. 2012; 27:161–5.
Article44. Wlodzimirow KA, Abu-Hanna A, Slabbekoorn M, Chamuleau RA, Schultz MJ, Bouman CS. A comparison of RIFLE with and without urine output criteria for acute kidney injury in critically ill patients. Crit Care. 2012; 16:R200.
Article45. Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015; 26:2231–8.
Article46. Qin JP, Yu XY, Qian CY, Li SS, Qin TH, Chen EZ, et al. Value of kidney disease improving global outcomes urine output criteria in critically ill patients: a secondary analysis of a multicenter prospective cohort study. Chin Med J (Engl). 2016; 129:2050–7.47. Bihari S, Prakash S, Bersten AD. Post resusicitation fluid boluses in severe sepsis or septic shock: prevalence and efficacy (price study). Shock. 2013; 40:28–34.48. Bihari S, Teubner DJ, Prakash S, Beatty T, Morphett M, Bellomo R, et al. Fluid bolus therapy in emergency department patients: indications and physiological changes. Emerg Med Australas. 2016; 28:531–7.
Article49. Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008; 12:169.
Article50. Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, et al. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008; 12:R74.
Article51. Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, et al. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005; 18:54–60.52. Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009; 76:422–7.
Article53. Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010; 14:R82.
Article54. Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004; 66:1613–21.
Article55. Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network. Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011; 6:966–73.
Article56. Liu KD, Thompson BT, Ancukiewicz M, Steingrub JS, Douglas IS, Matthay MA, et al. Acute kidney injury in patients with acute lung injury: impact of fluid accumulation on classification of acute kidney injury and associated outcomes. Crit Care Med. 2011; 39:2665–71.
Article57. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006; 354:2564–75.
Article58. RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, et al. An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med. 2012; 40:1753–60.59. Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013; 17:R14.
Article60. Garzotto F, Ostermann M, Martin-Langerwerf D, Sanchez-Sanchez M, Teng J, Robert R, et al. The Dose Response Multicentre Investigation on Fluid Assessment (DoReMIFA) in critically ill patients. Crit Care. 2016; 20:196.
Article61. Neyra JA, Li X, Canepa-Escaro F, Adams-Huet B, Toto RD, Yee J, et al. Cumulative fluid balance and mortality in septic patients with or without acute kidney injury and chronic kidney disease. Crit Care Med. 2016; 44:1891–900.
Article62. Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Kashani K. The impact of fluid balance on diagnosis, staging and prediction of mortality in critically ill patients with acute kidney injury. J Nephrol. 2016; 29:221–7.
Article63. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39:259–65.
Article64. Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, et al. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013; 17:R278.
Article65. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008; 134:172–8.
Article66. Wong BT, Chan MJ, Glassford NJ, Martensson J, Bion V, Chai SY, et al. Mean arterial pressure and mean perfusion pressure deficit in septic acute kidney injury. J Crit Care. 2015; 30:975–81.
Article67. Okusa MD. The changing pattern of acute kidney injury: from one to multiple organ failure. Contrib Nephrol. 2010; 165:153–8.
Article68. Virzì G, Day S, de Cal M, Vescovo G, Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014; 18:201.
Article69. Paul E, Bailey M, Kasza J, Pilcher D. The ANZROD model: better benchmarking of ICU outcomes and detection of outliers. Crit Care Resusc. 2016; 18:25–36.70. Higgins TL, Teres D, Nathanson B. Outcome prediction in critical care: the Mortality Probability Models. Curr Opin Crit Care. 2008; 14:498–505.
Article71. Higgins TL, Teres D, Copes WS, Nathanson BH, Stark M, Kramer AA. Assessing contemporary intensive care unit outcome: an updated Mortality Probability Admission Model (MPM0-III). Crit Care Med. 2007; 35:827–35.
Article72. Higgins TL, Kramer AA, Nathanson BH, Copes W, Stark M, Teres D. Prospective validation of the intensive care unit admission Mortality Probability Model (MPM0-III). Crit Care Med. 2009; 37:1619–23.
Article73. Lemeshow S, Teres D, Klar J, Avrunin JS, Gehlbach SH, Rapoport J. Mortality Probability Models (MPM II) based on an international cohort of intensive care unit patients. JAMA. 1993; 270:2478–86.
Article74. Le Gall JR, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D, et al. A simplified acute physiology score for ICU patients. Crit Care Med. 1984; 12:975–7.75. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993; 270:2957–63.
Article76. Capuzzo M, Moreno RP, Le Gall JR. Outcome prediction in critical care: the simplified acute physiology score models. Curr Opin Crit Care. 2008; 14:485–90.
Article77. Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005; 31:1345–55.78. Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, et al. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005; 31:1336–44.79. Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981; 9:591–7.
Article80. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985; 13:818–29.81. Pilcher DV, Hoffman T, Thomas C, Ernest D, Hart GK. Risk-adjusted continuous outcome monitoring with an EWMA chart: could it have detected excess mortality among intensive care patients at Bundaberg Base Hospital? Crit Care Resusc. 2010; 12:36–41.82. Paul E, Bailey M, Van Lint A, Pilcher V. Performance of APACHE III over time in Australia and New Zealand: a retrospective cohort study. Anaesth Intensive Care. 2012; 40:980–94.
Article83. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006; 34:1297–310.
Article84. Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013; 28:935–41.
Article85. Lee H, Shon YJ, Kim H, Paik H, Park HP. Validation of the APACHE IV model and its comparison with the APACHE II, SAPS 3, and Korean SAPS 3 models for the prediction of hospital mortality in a Korean surgical intensive care unit. Korean J Anesthesiol. 2014; 67:115–22.
Article86. Mann SL, Marshall MR, Woodford BJ, Holt A, Williams AB. Predictive performance of Acute Physiological and Chronic Health Evaluation releases II to IV: a single New Zealand centre experience. Anaesth Intensive Care. 2012; 40:479–89.
Article87. Wong RS, Ismail NA, Tan CC. An external independent validation of APACHE IV in a Malaysian intensive care unit. Ann Acad Med Singapore. 2015; 44:127–32.88. Pilcher D, Paul E, Bailey M, Huckson S. The Australian and New Zealand Risk of Death (ANZROD) model: getting mortality prediction right for intensive care units. Crit Care Resusc. 2014; 16:3–4.89. Australian and New Zealand Intensive Care Society. CORE–adult patient database (APD) [Internet]. Carlton: Australian and New Zealand Intensive Care Society;2016. [cited 2017 May 12]. Available from: http://www.anzics.com.au/pages/CORE/apd.aspx.90. Harrison DA, Parry GJ, Carpenter JR, Short A, Rowan K. A new risk prediction model for critical care: the Intensive Care National Audit & Research Centre (ICNARC) model. Crit Care Med. 2007; 35:1091–8.91. Bagshaw SM, Cruz DN. Fluid overload as a biomarker of heart failure and acute kidney injury. Contrib Nephrol. 2010; 164:54–68.
Article92. O’Connor ME, Prowle JR. Fluid overload. Crit Care Clin. 2015; 31:803–21.
Article93. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010; 15:309–34.
Article94. Schortgen F, Charles-Nelson A, Bouadma L, Bizouard G, Brochard L, Katsahian S. Respective impact of lowering body temperature and heart rate on mortality in septic shock: mediation analysis of a randomized trial. Intensive Care Med. 2015; 41:1800–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 6 Cases of Exfoliatiave Dermatitis
- Influence of Daily Fluid Balance prior to Continuous Renal Replacement Therapy on Outcomes in Critically Ill Patients
- The stress Buffering Effects of Medical Information Among: The Diabetic Patients
- The Factors associated with Oliguria after Indomethacin Administration in Preterm Infants with Patent Ductus Arteriosus
- Fluid Therapy and Transfusion on Mechanical Ventilation