Transl Clin Pharmacol.
2016 Mar;24(1):13-21. 10.12793/tcp.2016.24.1.13.
Brief introduction to current pharmacogenomics research tools
- Affiliations
-
- 1Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan 47392, Korea. hosuegi@gmail.com
- 2Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Busan 47392, Korea.
- KMID: 2383595
- DOI: http://doi.org/10.12793/tcp.2016.24.1.13
Abstract
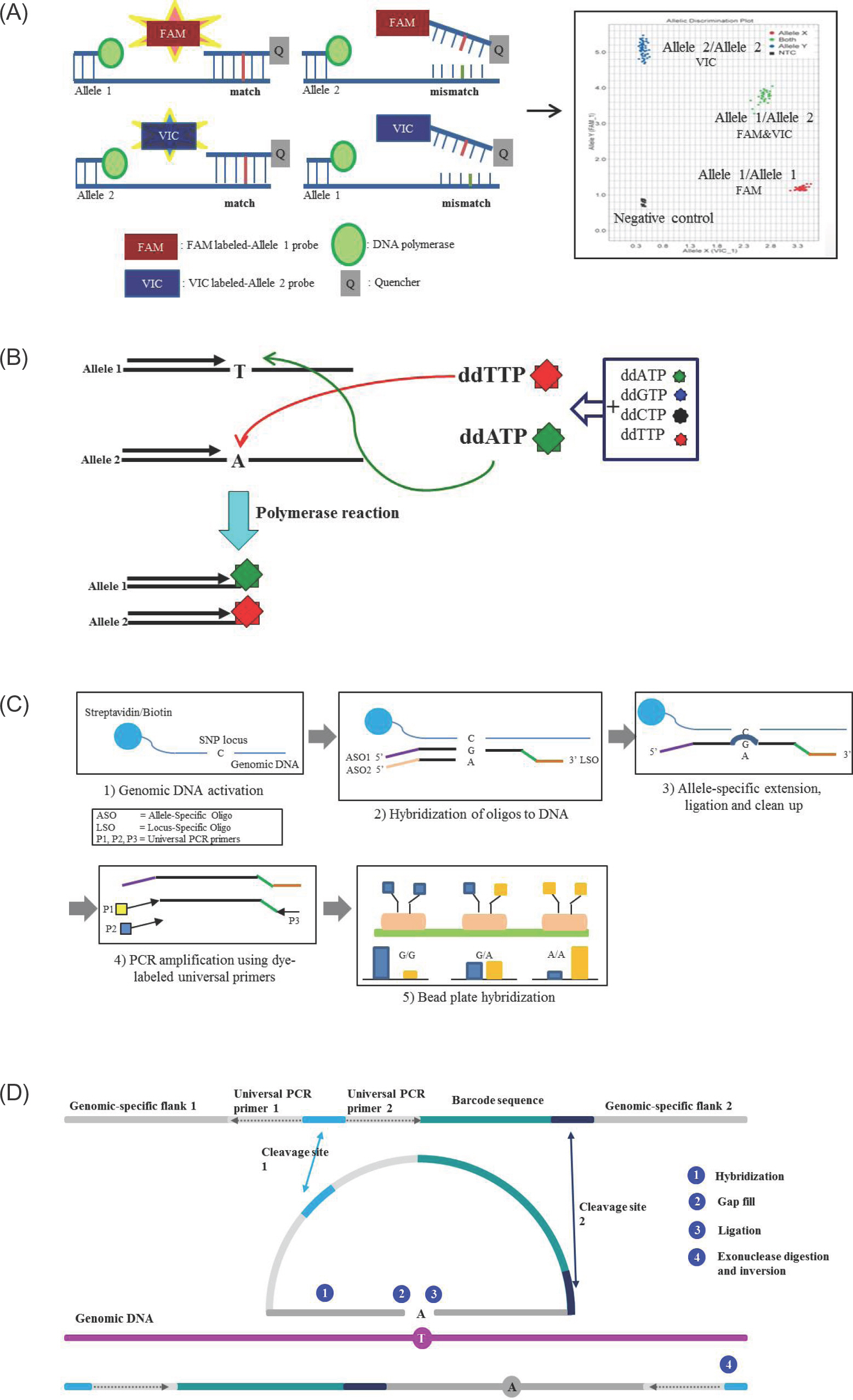
- There is increasing interest in the application of personalized therapy to healthcare to increase the effectiveness of and reduce the adverse reactions to treatment. Pharmacogenomics is a core element in personalized therapy and pharmacogenomic research is a growing field. Understanding pharmacogenomic research tools enables better design, conduct, and analysis of pharmacogenomic studies, as well as interpretation of pharmacogenomic results. This review provides a general and brief introduction to pharmacogenomics research tools, including genotyping technology, web-based genome browsers, and software for haplotype analysis.
Keyword
Figure
Reference
-
References
1. Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011; 364:1144–1153.
Article2. Mooney SD. Progress towards the integration of pharmacogenomics in practice. Hum Genet. 2015; 134:459–465.
Article3. Flynn AA. Pharmacogenetics: practices and opportunities for study design and data analysis. Drug Discov Today. 2011; 16:862–866.
Article4. Roden DM, George AL Jr. The genetic basis of variability in drug responses. Nat Rev Drug Discov. 2002; 1:37–44.
Article5. Lee JK, Part3 Disease association study. Genetic variation and Diseases (Language in Korean). 2nd ed.Seoul;2010. p. 211–262.6. Kim S, Misra A. SNP genotyping: technologies and biomedical applications. Annu Rev Biomed Eng. 2007; 9:289–320.
Article7. NwanKwo DC AM. Restriction enzymes and their uses in specific sequencing to produce predictable fragment of DNA making genetic engineering simply. Journal of Pharmaceutical Research and Opinion. 2011; 5:148–152.8. Nobile C, Romeo G. Partial digestion with restriction enzymes of ultraviolet-irradiated human genomic DNA: a method for identifying restriction site polymorphisms. Genomics. 1988; 3:272–274.
Article9. Koch WH. Technology platforms for pharmacogenomic diagnostic assays. Nat Rev Drug Discov. 2004; 3:749–761.
Article10. Livak KJ. SNP genotyping by the 5'-nuclease reaction. Methods Mol Biol. 2003; 212:129–147.
Article11. Frederickson RM. Fluidigm. Biochips get indoor plumbing. Chem Biol. 2002; 9:1161–1162.12. Hsiao SJ, Rai AJ, Multiplexed Pharmacogenetic Assays for SNP Genotyping: Tools and Techniques for Individualizing Patient Therapy. In: Dr. Des-pina Sanoudou (ed) Clinical Applications. 1st ed.Yan An Road (West), Shanghai;2012. p. 35–54.13. Nikolausz M, Chatzinotas A, Táncsics A, Imfeld G, Kästner M. The single-nucleotide primer extension (SNuPE) method for the multiplex detection of various DNA sequences: from detection of point mutations to microbial ecology. Biochem Soc Trans. 2009; 37:454–459. doi: 10.1042/BST0370454.
Article14. Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001; 11:3–11.
Article15. Zhou Z, Poe AC, Limor J, Grady KK, Goldman I, McCollum AM, et al. Pyrosequencing, a high-throughput method for detecting single nucleotide polymorphisms in the dihydrofolate reductase and dihydropteroate synthetase genes of Plasmodium falciparum. J Clin Microbiol. 2006; 44:3900–3910.16. Hurst CD, Zuiverloon TC, Hafner C, Zwarthoff EC, Knowles MA. A SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res Notes. 2009; 2:66. doi: 10.1186/1756-0500-2-66.
Article17. Syrmis MW, Moser RJ, Kidd TJ, Hunt P, Ramsay KA, Bell SC, et al. High-throughput single389 nucleotide polymorphism-based typing of shared Pseudomonas aeruginosa strains in cystic fibrosis patients using the Sequenom iPLEX platform. J Med Microbiol. 2013; 62:734–740.18. Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009. ;Chapter 2: Unit 2.12.doi: 10.1002/0471142905.hg0212s60.
Article19. Tian HL, Wang FG, Zhao JR, Yi HM, Wang L, Wang R, et al. Development of maizeSNP3072, a high-throughput compatible SNP array, for DNA fingerprinting identification of Chinese maize varieties. Mol Breed. 2015; 35:136.
Article20. Dalma-Weiszhausz DD, Warrington J, Tanimoto EY, Miyada CG. The affymetrix GeneChip platform: an overview. 398 Methods Enzymol. 2006; 410:3–28.21. Spudich GM, Fernández-Suárez XM. Touring Ensembl: a practical guide to genome browsing. BMC Genomics. 2010; 11:295. doi: 10.1186/1471-2164-11-295.
Article22. Wang J, Kong L, Gao G, Luo J. A brief introduction to web-based genome browsers. Brief Bioinform. 2013; 14:131–143.
Article23. Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014; 42:D980–D985. doi: 10.1093/nar/gkt1113.
Article24. Rosenbloom KR, Armstrong J, Barber GP, Casper J, Clawson H, Diekhans M, et al. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015; 43:D670–D681.25. Harel A, Inger A, Stelzer G, Strichman-Almashanu L, Dalah I, Safran M, et al. GIFtS: annotation landscape analysis with GeneCards. BMC Bioinformatics. 2009; 10:348.
Article26. Safran M, Dalah I, Alexander J, Rosen N, Iny Stein T, Shmoish M, et al. GeneCards Version 3: the human gene integrator. Database (Oxford). 2010. baq020. doi: 010.1093/database/baq1020.
Article27. Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic Acids Res. 2014; 42:D749–D755.
Article28. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012; 491:56–65.
Article29. Consortium IH. The International HapMap Project. Nature. 2003; 426:789–796.
Article30. Consortium IH. A haplotype map of the human genome. Nature. 2005; 437:1299–1320.
Article31. Clark AG. Inference of haplotypes from PCR-amplified samples of diploid populations. Mol Biol Evol. 1990; 7:111–122.32. Barrett JC. Haploview: Visualization and analysis of SNP genotype data. Cold Spring Harb Protoc. 2009; 2009:pdb ip71.doi: 10.1101/pdb.ip1171.
Article33. Hawley ME, Kidd KK. HAPLO: a program using the EM algorithm to estimate the frequencies of multisite haplotypes. J Hered. 1995; 86:409–411.
Article34. Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995; 12:921–927.35. Clayton D. A generalization of the transmission/disequilibrium test for uncertain-haplotype transmission. Am J Hum Genet. 1999; 65:1170–1177.
Article36. Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001; 68:978–989.
Article37. Morris AP, Whittaker JC, Balding DJ. Fine-scale mapping of disease loci via shattered coalescent modeling of genealogies. Am J Hum Genet. 2002; 70:686–707.
Article38. Niu T, Qin ZS, Xu X, Liu JS. Bayesian haplotype inference for multiple linked single434 nucleotide polymorphisms. Am J Hum Genet. 2002; 70:157–169.39. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005; 21:263–265.
Article40. Stephens M, Donnelly P. A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet. 2003; 73:1162–1169.
Article41. Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am J Hum Genet. 2005; 76:449–462.
Article42. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81:559–575.
Article