J Pathol Transl Med.
2015 May;49(3):218-229. 10.4132/jptm.2015.04.15.
Pathology-MRI Correlation of Hepatocarcinogenesis: Recent Update
- Affiliations
-
- 1Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. medimash@gmail.com
- 2Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2381380
- DOI: http://doi.org/10.4132/jptm.2015.04.15
Abstract
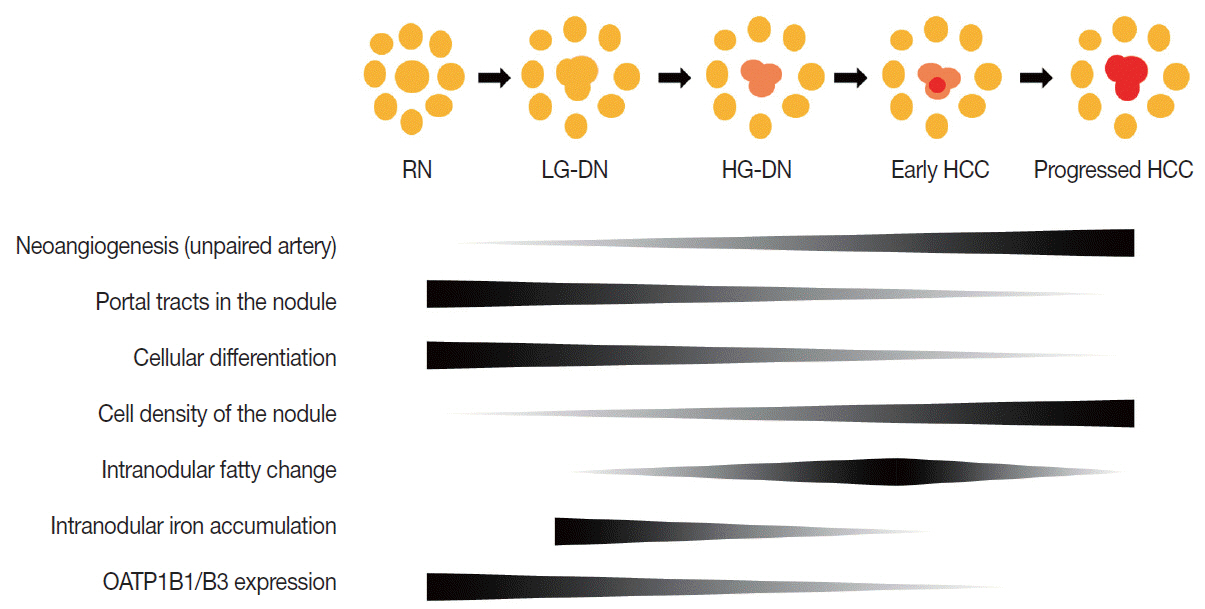
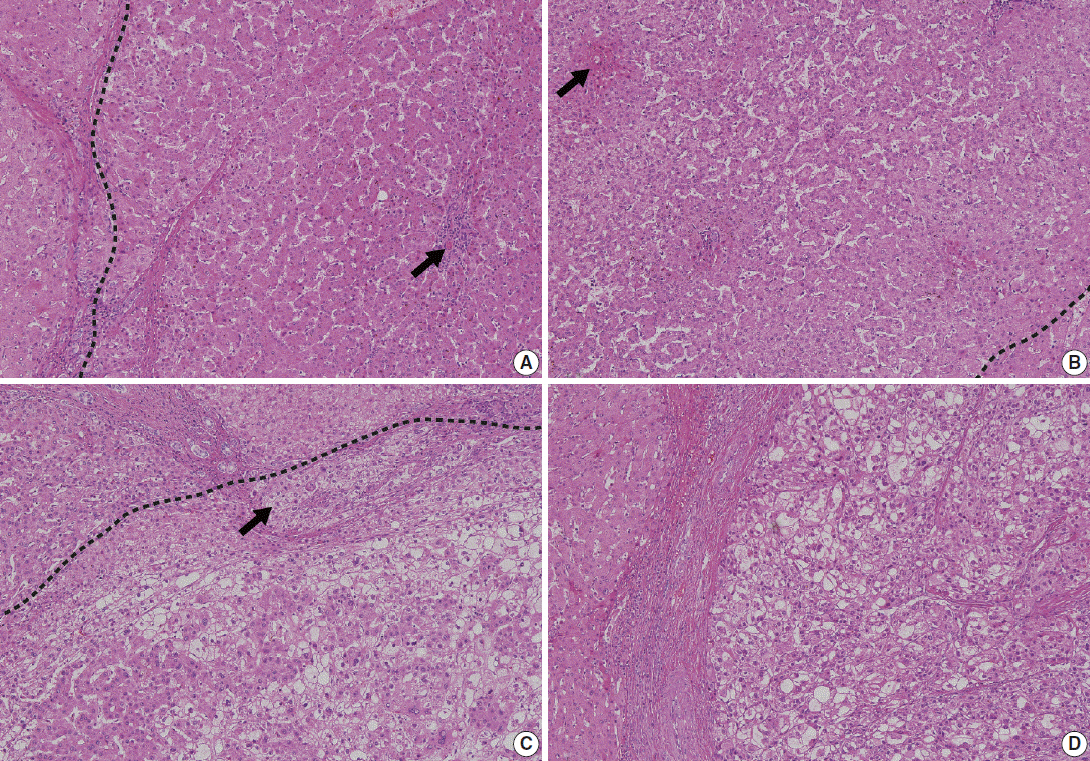
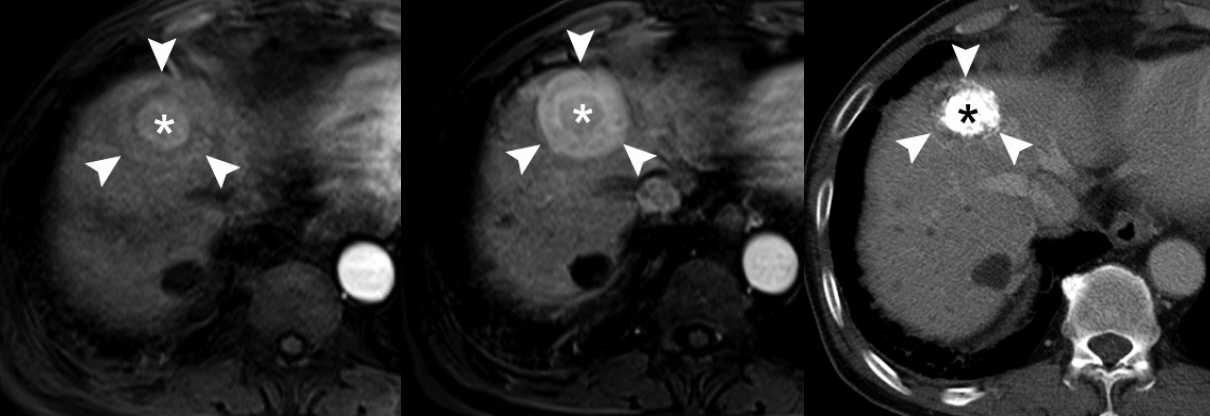
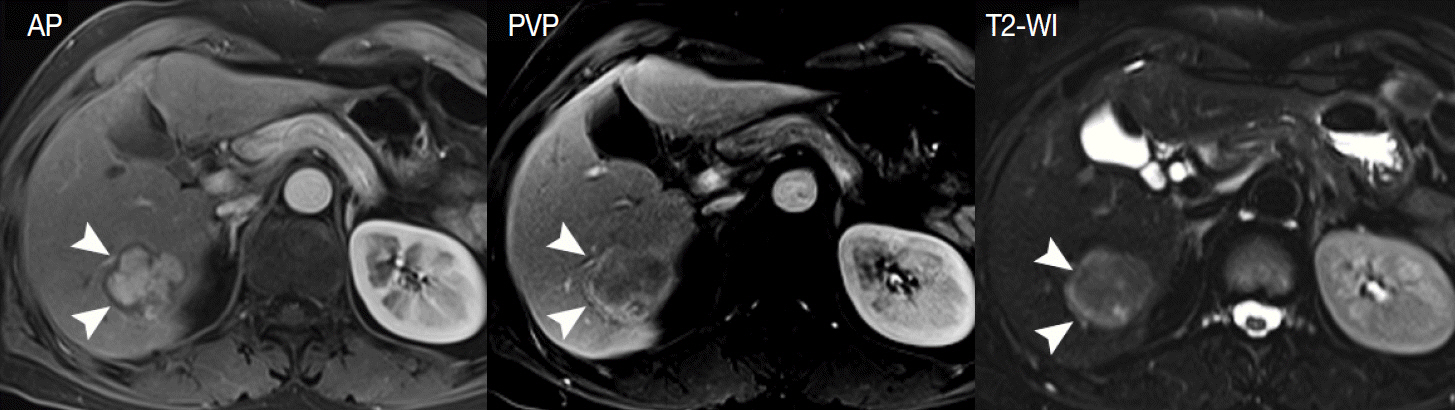
- Understanding the important alterations during hepatocarcinogenesis as well as the characteristic magnetic resonance imaging (MRI) and histopathological features will be helpful for managing patients with chronic liver disease and hepatocellular carcinoma. Recent advances in MRI techniques, such as fat/iron quantification, diffusion-weighted images, and gadoxetic acid-enhanced MRI, have greatly enhanced our understanding of hepatocarcinogenesis.
Figure
Reference
-
1. Song P, Tang W, Tamura S, et al. The management of hepatocellular carcinoma in Asia: a guideline combining quantitative and qualitative evaluation. Biosci Trends. 2010; 4:283–7.2. Kudo M, Han KH, Kokudo N, et al. Liver Cancer Working Group report. Jpn J Clin Oncol. 2010; 40 Suppl 1:i19–27.
Article3. Lee SH, Choi HC, Jeong SH, et al. Hepatocellular carcinoma in older adults: clinical features, treatments, and survival. J Am Geriatr Soc. 2011; 59:241–50.
Article4. Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009; 44 Suppl 19:112–8.
Article5. Yoo HJ, Lee JM, Lee MW, et al. Hepatocellular carcinoma in cirrhotic liver: double-contrast-enhanced, high-resolution 3.0T-MR imaging with pathologic correlation. Invest Radiol. 2008; 43:538–46.
Article6. Choi BI, Lee JM. Advancement in HCC imaging: diagnosis, staging and treatment efficacy assessments: imaging diagnosis and staging of hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2010; 17:369–73.7. Henninger B, Zoller H, Rauch S, et al. Automated two-point dixon screening for the evaluation of hepatic steatosis and siderosis: comparison with R2*-relaxometry and chemical shift-based sequences. Eur Radiol. 2015; 25:1356–65.
Article8. Suh CH, Kim KW, Kim GY, Shin YM, Kim PN, Park SH. The diagnostic value of Gd-EOB-DTPA-MRI for the diagnosis of focal nodular hyperplasia: a systematic review and meta-analysis. Eur Radiol. 2015; 25:950–60.
Article9. Krinsky G. Imaging of dysplastic nodules and small hepatocellular carcinomas: experience with explanted livers. Intervirology. 2004; 47:191–8.
Article10. Fournier LS, Cuenod CA, de Bazelaire C, et al. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol. 2004; 14:2125–33.
Article11. Roncalli M, Terracciano L, Di Tommaso L, et al. Liver precancerous lesions and hepatocellular carcinoma: the histology report. Dig Liver Dis. 2011; 43 Suppl 4:S361–72.
Article12. Ishigami K, Yoshimitsu K, Nishihara Y, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009; 250:435–43.
Article13. Kutami R, Nakashima Y, Nakashima O, Shiota K, Kojiro M. Pathomorphologic study on the mechanism of fatty change in small hepatocellular carcinoma of humans. J Hepatol. 2000; 33:282–9.
Article14. Pascale RM, De Miglio MR, Muroni MR, et al. Transferrin and transferrin receptor gene expression and iron uptake in hepatocellular carcinoma in the rat. Hepatology. 1998; 27:452–61.
Article15. Holmstrom P, Gåfvels M, Eriksson LC, et al. Expression of iron regulatory genes in a rat model of hepatocellular carcinoma. Liver Int. 2006; 26:976–85.
Article16. Libbrecht L, Craninx M, Nevens F, Desmet V, Roskams T. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with non-cirrhotic and cirrhotic chronic viral hepatitis. Histopathology. 2001; 39:66–73.
Article17. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014; 272:635–54.
Article18. International Working Party. Terminology of nodular hepatocellular lesions. Hepatology. 1995; 22:983–93.19. International Consensus Group for Hepatocellular Neoplasia; The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology. 2009; 49:658–64.20. Park YN. Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med. 2011; 135:704–15.
Article21. Okuda K, Peters RL, Simson IW. Gross anatomic features of hepatocellular carcinoma from three disparate geographic areas: proposal of new classification. Cancer. 1984; 54:2165–73.
Article22. Korean Liver Cancer Study Group. Pathology for prediction of liver. General rules for the study of primary liver cancer. Seoul: Korean Liver Cancer Study Group;2004. p. 39–40.23. Choi BI, Lee GK, Kim ST, Han MC. Mosaic pattern of encapsulated hepatocellular carcinoma: correlation of magnetic resonance imaging and pathology. Gastrointest Radiol. 1990; 15:238–40.
Article24. Demirjian A, Peng P, Geschwind JF, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011; 15:2089–97.
Article25. Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503.26. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–2.
Article27. Lee JM, Choi BI. Hepatocellular nodules in liver cirrhosis: MR evaluation. Abdom Imaging. 2011; 36:282–9.
Article28. Zech CJ, Reiser MF, Herrmann KA. Imaging of hepatocellular carcinoma by computed tomography and magnetic resonance imaging: state of the art. Dig Dis. 2009; 27:114–24.
Article29. Bitar R, Leung G, Perng R, et al. MR pulse sequences: what every radiologist wants to know but is afraid to ask. Radiographics. 2006; 26:513–37.
Article30. Kang BK, Yu ES, Lee SS, et al. Hepatic fat quantification: a prospective comparison of magnetic resonance spectroscopy and analysis methods for chemical-shift gradient echo magnetic resonance imaging with histologic assessment as the reference standard. Invest Radiol. 2012; 47:368–75.31. Ayyappan AP, Jhaveri KS. CT and MRI of hepatocellular carcinoma: an update. Expert Rev Anticancer Ther. 2010; 10:507–19.
Article32. Sun HY, Lee JM, Shin CI, et al. Gadoxetic acid-enhanced magnetic resonance imaging for differentiating small hepatocellular carcinomas (< or =2 cm in diameter) from arterial enhancing pseudolesions: special emphasis on hepatobiliary phase imaging. Invest Radiol. 2010; 45:96–103.33. Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009; 193:14–27.
Article34. Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology. 2010; 254:47–66.
Article35. Bartolozzi C, Battaglia V, Bozzi E. HCC diagnosis with liver-specific MRI: close to histopathology. Dig Dis. 2009; 27:125–30.36. Hayashi M, Matsui O, Ueda K, et al. Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol. 1999; 172:969–76.
Article37. Choi BI. Hepatocarcinogenesis in liver cirrhosis: imaging diagnosis. J Korean Med Sci. 1998; 13:103–16.
Article38. Han JK, Eun HW, Kim SH. Radiologic findings of dysplastic nodule. Korean J Hepatol. 2008; 14:231–4.
Article39. Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics. 2008; 28:747–69.
Article40. Trevisani F, Cantarini MC, Wands JR, Bernardi M. Recent advances in the natural history of hepatocellular carcinoma. Carcinogenesis. 2008; 29:1299–305.
Article41. Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013; 87:227–47.
Article42. Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002; 31:339–46.
Article43. Ichikawa T, Sano K, Morisaka H. Diagnosis of pathologically early HCC with EOB-MRI: experiences and current consensus. Liver Cancer. 2014; 3:97–107.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathology of Hepatocellular Carcinoma: Recent Update
- Role of Angiogenic Factors during the Hepatocarcinogenesis
- Recent Update of Pathology of the Pancreatic Neuroendocrine Tumor
- The dual role of transforming growth factor-beta signatures in human B viral multistep hepatocarcinogenesis: early and late responsive genes
- Advances in hepatocellular carcinoma: hepatocarcinogenesis, role of exosomal noncoding RNAs, and diagnostic pathology