Allergy Asthma Immunol Res.
2017 Jul;9(4):288-298. 10.4168/aair.2017.9.4.288.
In Vitro Diagnostic Testing for Antibiotic Allergy
- Affiliations
-
- 1Allergy Unit, IBIMA-Regional University Hospital of Malaga-UMA, Málaga, Spain. mjtorresj@ibima.eu
- 2Research Laboratory, IBIMA-Regional University Hospital of Malaga-UMA, Málaga, Spain.
- 3Andalusian Center for Nanomedicine and Biotechnology-BIONAND, Málaga, Spain.
- KMID: 2378191
- DOI: http://doi.org/10.4168/aair.2017.9.4.288
Abstract
- Allergy to antibiotics is an important worldwide problem, with an estimated prevalence of up to 10% of the population. Reaction patterns for different antibiotics have changed in accordance with consumption trends. Most of the allergic reactions to antibiotics have been reported for betalactams, followed by quinolones and macrolides and, to a lesser extent, to others, such as metronidazole clindamycin and sulfonamides. The diagnostic procedure includes a detailed clinical history, which is not always possible and can be unreliable. This is usually followed by in vivo, skin, and drug provocation tests. These are not recommended for severe, potentially lifethreaten reactions or for drugs that are known to produce a high rate of false positive results. Given the limitations of in vivo tests, in vitro test can be helpful for diagnosis, despite having suboptimal sensitivity. The most highly employed techniques for diagnosing immediate reactions to antibiotics are immunoassays and basophil activation tests, while lymphocyte transformation tests are more commonly used to diagnose non-immediate reactions. In this review, we describe different in vitro techniques employed to diagnose antibiotic allergy.
Keyword
MeSH Terms
-
Anti-Bacterial Agents
Basophils
Clindamycin
Diagnosis
Diagnostic Tests, Routine*
Drug Hypersensitivity
Hypersensitivity*
Immunoassay
In Vitro Techniques*
Lymphocyte Activation
Macrolides
Metronidazole
Prevalence
Quinolones
Skin
Sulfonamides
Anti-Bacterial Agents
Clindamycin
Macrolides
Metronidazole
Quinolones
Sulfonamides
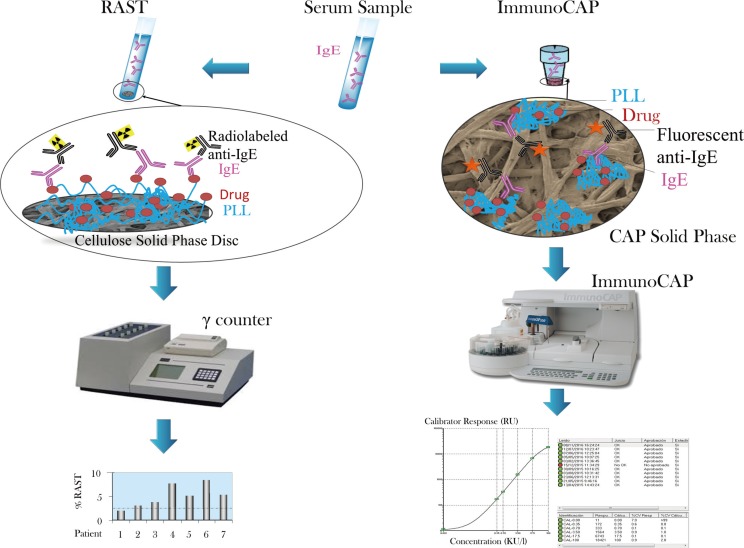
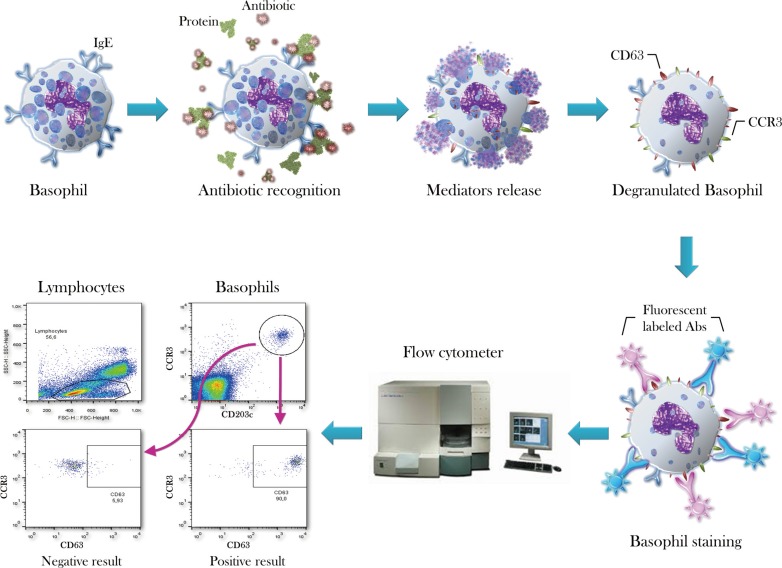
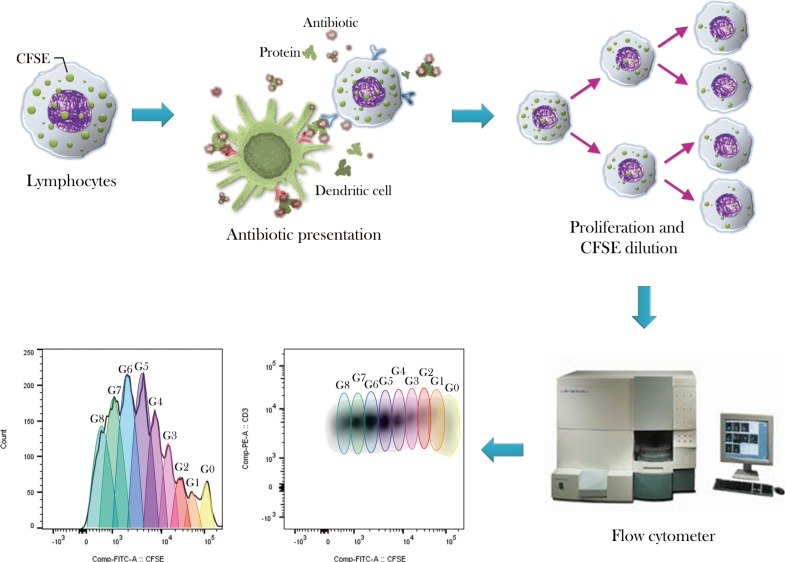
Figure
Cited by 1 articles
-
Proper Cut-off Levels of Serum Specific IgE to Cefaclor for Patients with Cefaclor Allergy
Young-Hee Nam, So-Hee Lee, Hyo-In Rhyou, Young-Soo Lee, Seung-Hee Park, Young-Hee Lee, Yoo-Seob Shin, Hae-Sim Park, Young-Min Ye
Yonsei Med J. 2018;59(8):968-974. doi: 10.3349/ymj.2018.59.8.968.
Reference
-
1. Romano A, Warrington R. Antibiotic allergy. Immunol Allergy Clin North Am. 2014; 34:489–506. PMID: 25017674.
Article2. Torres MJ, Montañez MI, Ariza A, Salas M, Fernandez TD, Barbero N, et al. The role of IgE recognition in allergic reactions to amoxicillin and clavulanic acid. Clin Exp Allergy. 2016; 46:264–274. PMID: 26662186.
Article3. Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014; 14:742–750. PMID: 25022435.
Article4. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005; 5:309–316. PMID: 15985812.
Article5. Doña I, Blanca-López N, Torres MJ, García-Campos J, García-Núñez I, Gómez F, et al. Drug hypersensitivity reactions: response patterns, drug involved, and temporal variations in a large series of patients. J Investig Allergol Clin Immunol. 2012; 22:363–371.6. Romano A, Torres MJ, Castells M, Sanz ML, Blanca M. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011; 127:S67–S73. PMID: 21354502.
Article7. Torres MJ, Salas M, Ariza A, Fernández TD. Understanding the mechanisms in accelerated drug reactions. Curr Opin Allergy Clin Immunol. 2016; 16:308–314. PMID: 27285487.
Article8. Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003; 139:683–693. PMID: 14568857.
Article9. Blanca M, Mayorga C, Torres MJ, Warrington R, Romano A, Demoly P, et al. Side-chainspecific reactions to betalactams: 14 years later. Clin Exp Allergy. 2002; 32:192–197. PMID: 11929481.
Article10. Torres MJ, Blanca M. The complex clinical picture of beta-lactam hypersensitivity: penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med Clin North Am. 2010; 94:805–820. PMID: 20609864.11. Romano A, Piunti E, Di Fonso M, Viola M, Venuti A, Venemalm L. Selective immediate hypersensitivity to ceftriaxone. Allergy. 2000; 55:415–416. PMID: 10782539.
Article12. Torres MJ, Ariza A, Mayorga C, Doña I, Blanca-Lopez N, Rondon C, et al. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J Allergy Clin Immunol. 2010; 125:502–505.e2. PMID: 20159266.
Article13. Aranda A, Mayorga C, Ariza A, Doña I, Rosado A, Blanca-Lopez N, et al. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy. 2011; 66:247–254. PMID: 20722637.14. Schmid DA, Depta JP, Pichler WJ. T cell-mediated hypersensitivity to quinolones: mechanisms and crossreactivity. Clin Exp Allergy. 2006; 36:59–69. PMID: 16393267.
Article15. Blanca-López N, Ariza A, Doña I, Mayorga C, Montañez MI, Garcia-Campos J, et al. Hypersensitivity reactions to fluoroquinolones: analysis of the factors involved. Clin Exp Allergy. 2013; 43:560–567. PMID: 23600547.
Article16. Gruchalla RS. 10. Drug allergy. J Allergy Clin Immunol. 2003; 111:S548–S559. PMID: 12592301.
Article17. Schnyder B, Pichler WJ. Allergy to sulfonamides. J Allergy Clin Immunol. 2013; 131:256–257.e1-e5. PMID: 23265699.
Article18. Anne' S, Middleton E Jr, Reisman RE. Vancomycin anaphylaxis and successful desensitization. Ann Allergy. 1994; 73:402–404. PMID: 7978531.19. Bernedo N, González I, Gastaminza G, Audicana M, Fernández E, Muñoz D. Positive patch test in vancomycin allergy. Contact Dermatitis. 2001; 45:43. PMID: 11422272.
Article20. Sharif S, Goldberg B. Detection of IgE antibodies to bacitracin using a commercially available streptavidinlinked solid phase in a patient with anaphylaxis to triple antibiotic ointment. Ann Allergy Asthma Immunol. 2007; 98:563–566. PMID: 17601270.
Article21. Connolly M, McAdoo J, Bourke JF. Gentamicin-induced anaphylaxis. Ir J Med Sci. 2007; 176:317–318. PMID: 17724569.
Article22. Araújo L, Demoly P. Macrolides allergy. Curr Pharm Des. 2008; 14:2840–2862. PMID: 18991703.23. Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998; 37:833–838. PMID: 9865869.
Article24. Joshi N, Miller DQ. Doxycycline revisited. Arch Intern Med. 1997; 157:1421–1428. PMID: 9224219.
Article25. Brown RJ, Rother KI, Artman H, Mercurio MG, Wang R, Looney RJ, et al. Minocycline-induced drug hypersensitivity syndrome followed by multiple autoimmune sequelae. Arch Dermatol. 2009; 145:63–66. PMID: 19153345.
Article26. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014; 133:790–796. PMID: 24188976.27. Demoly P, Kropf R, Bircher A, Pichler WJ. Drug hypersensitivity: questionnaire. EAACI interest group on drug hypersensitivity. Allergy. 1999; 54:999–1003. PMID: 10505466.28. Torres MJ, Mayorga C, Leyva L, Guzman AE, Cornejo-García JA, Juarez C, et al. Controlled administration of penicillin to patients with a positive history but negative skin and specific serum IgE tests. Clin Exp Allergy. 2002; 32:270–276. PMID: 11929493.
Article29. Blanca M, Romano A, Torres MJ, Férnandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009; 64:183–193. PMID: 19133923.
Article30. Fernández TD, Ariza A, Palomares F, Montañez MI, Salas M, MartínSerrano A, et al. Hypersensitivity to fluoroquinolones: The expression of basophil activation markers depends on the clinical entity and the culprit fluoroquinolone. Medicine (Baltimore). 2016; 95:e3679. PMID: 27281069.31. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003; 58:854–863. PMID: 12911412.
Article32. Mayorga C, Celik G, Rouzaire P, Whitaker P, Bonadonna P, Rodrigues-Cernadas J, et al. In vitro tests for drug hypersensitivity reactions: an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2016; 71:1103–1134. PMID: 26991315.33. Blanca M, Mayorga C, Torres MJ, Reche M, Moya MC, Rodriguez JL, et al. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy. 2001; 56:862–870. PMID: 11551251.34. Garcia JJ, Blanca M, Moreno F, Vega JM, Mayorga C, Fernandez J, et al. Determination of IgE antibodies to the benzylpenicilloyl determinant: a comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J Clin Lab Anal. 1997; 11:251–257. PMID: 9292392.
Article35. Fontaine C, Mayorga C, Bousquet PJ, Arnoux B, Torres MJ, Blanca M, et al. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate β-lactam allergy. Allergy. 2007; 62:47–52. PMID: 17156341.
Article36. Montañez MI, Perez-Inestrosa E, Suau R, Mayorga C, Torres MJ, Blanca M. Dendrimerized cellulose as a scaffold for artificial antigens with applications in drug allergy diagnosis. Biomacromolecules. 2008; 9:1461–1466. PMID: 18435560.
Article37. Vida Y, Montañez MI, Collado D, Najera F, Ariza A, Blanca M, et al. Dendrimeric antigen-silica particle composites: an innovative approach for IgE quantification. J Mater Chem B Mater Biol Med. 2013; 1:3044–3050.
Article38. Mayorga C, Perez-Inestrosa E, Molina N, Montañez MI. Development of nanostructures in the diagnosis of drug hypersensitivity reactions. Curr Opin Allergy Clin Immunol. 2016; 16:300–307. PMID: 27257940.
Article39. Montañez MI, Mayorga C, Torres MJ, Blanca M, Perez-Inestrosa E. Methodologies to anchor dendrimeric nanoconjugates to solid phase: toward an efficient in vitro detection of allergy to β-lactam antibiotics. Nanomedicine. 2011; 7:682–685. PMID: 21839054.40. Ruiz-Sanchez AJ, Montañez MI, Mayorga C, Torres MJ, Kehr NS, Vida Y, et al. Dendrimer-modified solid supports: nanostructured materials with potential drug allergy diagnostic applications. Curr Med Chem. 2012; 19:4942–4954. PMID: 22963628.
Article41. Romano A, Guéant-Rodriguez RM, Viola M, Amoghly F, Gaeta F, Nicolas JP, et al. Diagnosing immediate reactions to cephalosporins. Clin Exp Allergy. 2005; 35:1234–1242. PMID: 16164453.
Article42. Soler M, Mesa-Antunez P, Estevez MC, Ruiz-Sanchez AJ, Otte MA, Sepulveda B, et al. Highly sensitive dendrimer-based nanoplasmonic biosensor for drug allergy diagnosis. Biosens Bioelectron. 2015; 66:115–123. PMID: 25460891.
Article43. Fernández TD, Torres MJ, Blanca-López N, Rodríguez-Bada JL, Gomez E, Canto G, et al. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy. 2009; 64:242–248. PMID: 19178404.
Article44. Sanz ML, Gamboa PM, Antépara I, Uasuf C, Vila L, Garcia-Avilés C, et al. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin Exp Allergy. 2002; 32:277–286. PMID: 11929494.
Article45. Johansson SG, Adédoyin J, van Hage M, Grönneberg R, Nopp A. False-positive penicillin immunoassay: an unnoticed common problem. J Allergy Clin Immunol. 2013; 132:235–237. PMID: 23270810.
Article46. Vultaggio A, Matucci A, Virgili G, Rossi O, Filì L, Parronchi P, et al. Influence of total serum IgE levels on the in vitro detection of beta-lactams-specific IgE antibodies. Clin Exp Allergy. 2009; 39:838–844. PMID: 19400911.47. Vultaggio A, Virgili G, Gaeta F, Romano A, Maggi E, Matucci A. High serum β-lactams specific/total IgE ratio is associated with immediate reactions to β-lactams antibiotics. PLoS One. 2015; 10:e0121857. PMID: 25880869.
Article48. Manfredi M, Severino M, Testi S, Macchia D, Ermini G, Pichler WJ, et al. Detection of specific IgE to quinolones. J Allergy Clin Immunol. 2004; 113:155–160. PMID: 14713922.
Article49. Ariza A, Montañez MI, Fernández TD, Perkins JR, Mayorga C. Cellular tests for the evaluation of drug hypersensitivity. Curr Pharm Des. 2016; 22(45):6773–6783. PMID: 27829334.
Article50. Hoffmann HJ, Santos AF, Mayorga C, Nopp A, Eberlein B, Ferrer M, et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy. 2015; 70:1393–1405. PMID: 26198455.
Article51. De Week AL, Sanz ML, Gamboa PM, Aberer W, Bienvenu J, Blanca M, et al. Diagnostic tests based on human basophils: more potentials and perspectives than pitfalls. II. Technical issues. J Investig Allergol Clin Immunol. 2008; 18:143–155.52. Ghannadan M, Hauswirth AW, Schernthaner GH, Müller MR, Klepetko W, Schatzl G, et al. Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol. 2002; 127:299–307. PMID: 12021549.
Article53. Abuaf N, Rostane H, Rajoely B, Gaouar H, Autegarden JE, Leynadier F, et al. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008; 38:921–928. PMID: 18331364.
Article54. Torres MJ, Padial A, Mayorga C, Fernández T, Sanchez-Sabate E, Cornejo-García JA, et al. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin Exp Allergy. 2004; 34:1768–1775. PMID: 15544603.
Article55. De Week AL, Sanz ML, Gamboa PM, Aberer W, Sturm G, Bilo MB, et al. Diagnosis of immediatetype beta-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: a multicenter study. J Investig Allergol Clin Immunol. 2009; 19:91–109.56. Torres MJ, Romano A, Blanca-Lopez N, Doña I, Canto G, Ariza A, et al. Immunoglobulin E-mediated hypersensitivity to amoxicillin: in vivo and in vitro comparative studies between an injectable therapeutic compound and a new commercial compound. Clin Exp Allergy. 2011; 41:1595–1601. PMID: 21812844.57. Torres MJ, Ariza A, Fernández J, Moreno E, Laguna JJ, Montañez MI, et al. Role of minor determinants of amoxicillin in the diagnosis of immediate allergic reactions to amoxicillin. Allergy. 2010; 65:590–596. PMID: 19968633.
Article58. Rouzaire P, Nosbaum A, Denis L, Bienvenu F, Bérard F, Cozon G, et al. Negativity of the basophil activation test in quinolone hypersensitivity: a breakthrough for provocation test decision-making. Int Arch Allergy Immunol. 2012; 157:299–302. PMID: 22041525.
Article59. Ben Said B, Berard F, Bienvenu J, Nicolas JF, Rozieres A. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy. 2010; 65:535–536. PMID: 19845576.
Article60. Mayorga C, Andreu I, Aranda A, Doña I, Montañez MI, Blanca-Lopez N, et al. Fluoroquinolone photodegradation influences specific basophil activation. Int Arch Allergy Immunol. 2013; 160:377–382. PMID: 23183272.
Article61. Harrabi S, Loiseau P, Dehenry J. A technic for human basophil degranulation. Allerg Immunol (Paris). 1987; 19:287–289. PMID: 3453139.62. Ebo DG, Verheecke G, Bridts CH, Mertens CH, Stevens WJ. Perioperative anaphylaxis from locally applied rifamycin SV and latex. Br J Anaesth. 2006; 96:738–741. PMID: 16698868.
Article63. Pineda F, Ariza A, Mayorga C, Arribas F, González-Mendiola R, Blanca-López N, et al. Role of histamine release test for the evaluation of patients with immediate hypersensitivity reactions to clavulanic acid. Int Arch Allergy Immunol. 2015; 168:233–240. PMID: 26894754.
Article64. Kleine Budde I, de Heer PG, van der Zee JS, Aalberse RC. The stripped basophil histamine release bioassay as a tool for the detection of allergen-specific IgE in serum. Int Arch Allergy Immunol. 2001; 126:277–285. PMID: 11815734.
Article65. Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome-alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013; 43:1027–1037. PMID: 23957338.66. Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007; 62:1439–1444. PMID: 17983378.
Article67. Polak ME, Belgi G, McGuire C, Pickard C, Healy E, Friedmann PS, et al. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br J Dermatol. 2013; 168:539–549. PMID: 23106791.68. Luque I, Leyva L, José Torres M, Rosal M, Mayorga C, Segura JM, et al. In vitro Tcell responses to betalactam drugs in immediate and nonimmediate allergic reactions. Allergy. 2001; 56:611–618. PMID: 11421918.69. Rodriguez-Pena R, Lopez S, Mayorga C, Antunez C, Fernandez TD, Torres MJ, et al. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to beta-lactams. J Allergy Clin Immunol. 2006; 118:949–956. PMID: 17030251.70. Sanchez-Quintero MJ, Torres MJ, Blazquez AB, Gómez E, Fernandez TD, Doña I, et al. Synergistic effect between amoxicillin and TLR ligands on dendritic cells from amoxicillin-delayed allergic patients. PLoS One. 2013; 8:e74198. PMID: 24066120.
Article71. Scherer K, Bircher AJ. Hypersensitivity reactions to fluoroquinolones. Curr Allergy Asthma Rep. 2005; 5:15–21. PMID: 15659258.
Article72. Campi P, Pichler WJ. Quinolone hypersensitivity. Curr Opin Allergy Clin Immunol. 2003; 3:275–281. PMID: 12865771.
Article73. Kalish RS, LaPorte A, Wood JA, Johnson KL. Sulfonamidereactive lymphocytes detected at very low frequency in the peripheral blood of patients with drug-induced eruptions. J Allergy Clin Immunol. 1994; 94:465–472. PMID: 8083451.
Article74. Fu M, Gao Y, Pan Y, Li W, Liao W, Wang G, et al. Recovered patients with StevensJohson syndrome and toxic epidermal necrolysis maintain longlived IFN-gamma and sFasL memory response. PLoS One. 2012; 7:e45516. PMID: 23029066.