Cancer Res Treat.
2017 Apr;49(2):454-463. 10.4143/crt.2016.259.
Efficacy of Letrozole as First-Line Treatment of Postmenopausal Women with Hormone Receptor–Positive Metastatic Breast Cancer in Korea
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 3Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. ktyongmd@gmail.com moisa@snu.ac.kr
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2378118
- DOI: http://doi.org/10.4143/crt.2016.259
Abstract
- PURPOSE
Letrozole showed efficacy and generally favorable toxicities, along with the convenience of oral administration in postmenopausal patients with hormone receptor (HR)-positive metastatic breast cancer (MBC). To the best of our knowledge, there have been no reports of the clinical outcomes in Korean patients, although letrozole is widely used in practice. Therefore, this studywas conducted to affirm the efficacy and toxicities of letrozole in Korean patients.
MATERIALS AND METHODS
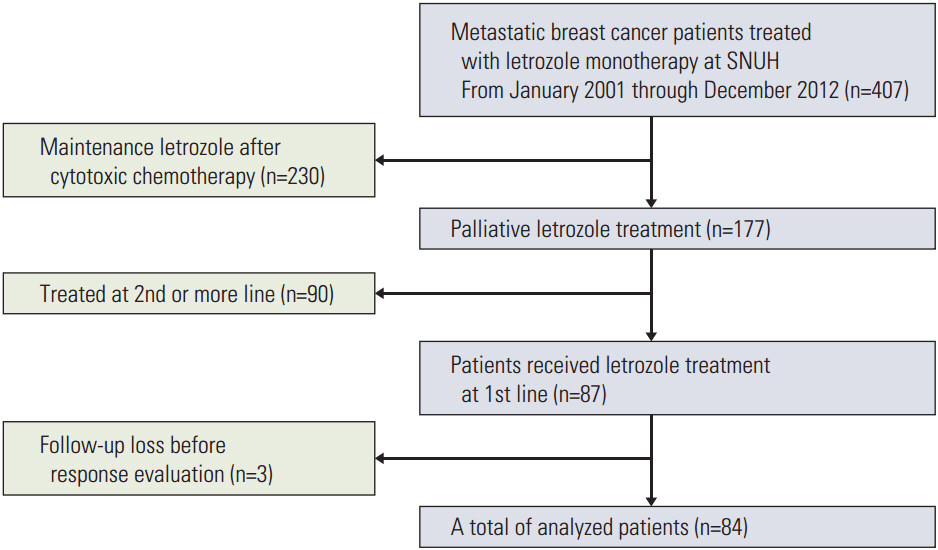
This study retrospectively analyzed 84 HR-positive MBC patients who had been treated with letrozole from January 2001 to December 2012. Clinicopathological characteristics and treatment history were extracted from medicalrecords. All patients received 2.5 mg letrozole once a day until there were disease progressions or unacceptable toxicity. Progression-free survival (PFS) was the primary endpoint, and secondary endpoints were overall survival (OS), objective response rate (ORR), and toxicity.
RESULTS
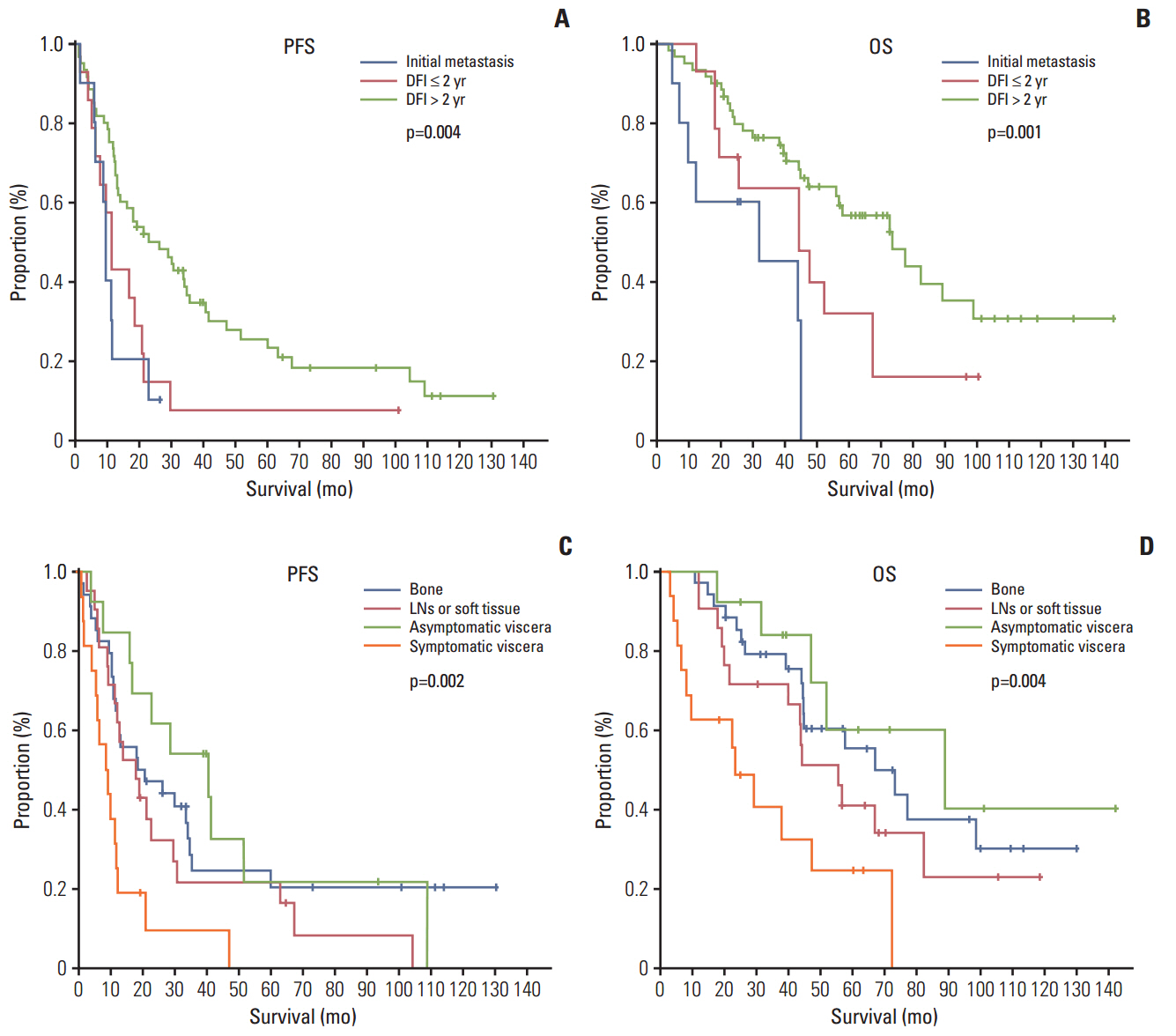
The median age of the subjects was 59.3 years. Letrozole treatment resulted in a median PFS of 16.8 months (95% confidence interval [CI], 9.8 to 23.8) and a median OS of 56.4 months (95% CI, 38.1 to 74.7). The ORR was 36.9% for the 84 patients with measurable lesions. Multivariate analysis revealed symptomatic visceral disease (hazard ratio, 3.437; 95% CI, 1.576 to 7.495; p=0.002) and a disease-free interval ≤ 2 years (hazard ratio, 2.697; 95% CI, 1.262 to 5.762; p=0.010) were independently associated with shorter PFS. However, sensitivity to adjuvant hormone treatment was not related to PFS. Letrozole was generally well tolerated.
CONCLUSION
Letrozole showed considerable efficacy and tolerability as a first-line treatment in postmenopausal patients with HR-positive MBC.
MeSH Terms
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Prediction of cancer incidence and mortality in Korea, 2013. Cancer Res Treat. 2013; 45:15–21.
Article3. Wilcken N, Hornbuckle J, Ghersi D. Chemotherapy alone versus endocrine therapy alone for metastatic breast cancer. Cochrane Database Syst Rev. 2003; (2):CD002747.
Article4. Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008; 26:4883–90.
Article5. Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000; 18:3748–57.
Article6. Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003; 21:2101–9.
Article7. Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009; 7:122–92.
Article8. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–50.
Article9. Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000; 18:3758–67.10. Kim Z, Min SY, Yoon CS, Jung KW, Ko BS, Kang E, et al. The basic facts of Korean breast cancer in 2012: results from a nationwide survey and breast cancer registry database. J Breast Cancer. 2015; 18:103–11.
Article11. Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001; 19:2596–606.
Article12. Gibson L, Lawrence D, Dawson C, Bliss J, et al. Aromatase inhibitors fortreatment of advanced breast cancerin postmenopausal women. Cochrane Database Syst Rev. 2009; (4):CD003370.
Article13. Kataja V, Castiglione M; ESMO Guidelines Working Group. Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008; 19 Suppl 2:ii11–3.
Article14. Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, et al. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res. 2008; 68:4910–8.
Article15. Murphy CG, Dickler MN. Endocrine resistance in hormoneresponsive breast cancer: mechanisms and therapeutic strategies. Endocr Relat Cancer. 2016; 23:R337–52.
Article16. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015; 16:25–35.
Article17. Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015; 373:209–19.
Article18. Mouridsen HT. Letrozole in advanced breast cancer: the PO25 trial. Breast Cancer Res Treat. 2007; 105 Suppl 1:19–29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Efficacy between First-Line Treatment Regimens for Patients with Hormone Receptor-Positive and HER2-Negative Metastatic Breast Cancer
- Personalized therapy for advanced breast cancer using molecular signatures
- An Effect of Letrozole on Gastric Cancer?
- Hormonal Changes during Extended Letrozole Treatment after Completion of 5 Years of Tamoxifen in Premenopausal Patients with Breast Cancer who Became Postmenopausal
- Endocrine Therapy for Breast Cancer