Diabetes Metab J.
2017 Apr;41(2):135-145. 10.4093/dmj.2017.41.2.135.
Addition of Ipragliflozin to Metformin Treatment in Korean Patients with Type 2 Diabetes Mellitus: Subgroup Analysis of a Phase 3 Trial
- Affiliations
-
- 1Department of Internal Medicine, Eulji General Hospital, Eulji University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- 3Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Endocrinology and Metabolism, Kyung Hee University Hospital at Gangdong, Kyung Hee University Medical Center, Seoul, Korea.
- 6Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Astellas Pharma Inc., Tokyo, Japan.
- 8Astellas Korea Inc., Seoul, Korea.
- 9Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bscha@yuhs.ac
- KMID: 2376776
- DOI: http://doi.org/10.4093/dmj.2017.41.2.135
Abstract
- BACKGROUND
This is a subgroup analysis of Korean patients from a phase 3 clinical trial investigating the efficacy and safety of ipragliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin.
METHODS
This multicenter, placebo-controlled, double-blind, parallel-group study was carried out between November 2011 and January 2013. Patients entered a 2-week placebo pretreatment period, followed by a 24-week treatment period with either ipragliflozin (50 mg/day) or placebo, while continuing metformin. Efficacy outcomes (glycosylated hemoglobin [HbA1c], fasting plasma glucose [FPG], and body weight) and safety outcomes (treatment-emergent adverse events [TEAEs]) were measured and compared between the two treatment groups for patients enrolled in all 18 study sites in Korea.
RESULTS
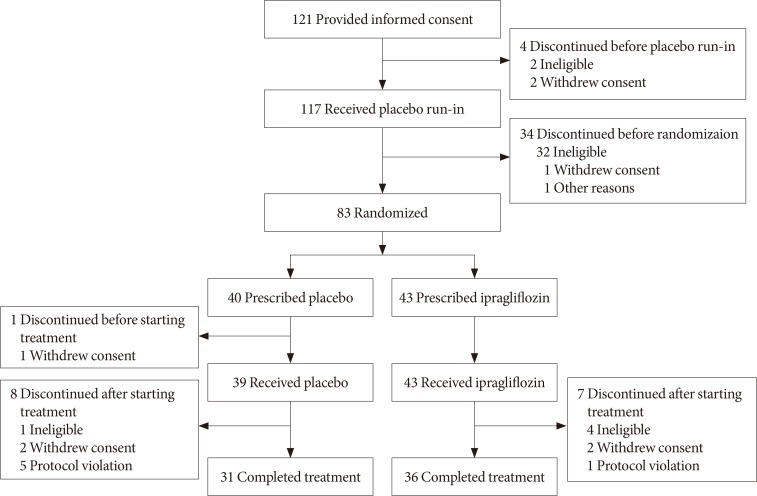
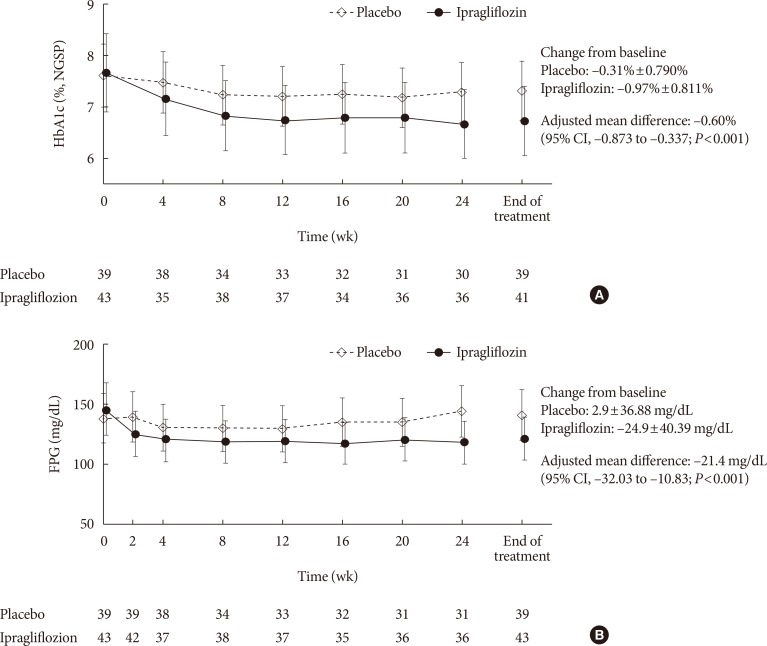
Eighty-two Korean patients received ipragliflozin (n=43) or placebo (n=39) during the study period. Mean changes in HbA1c levels from baseline to the end of treatment were -0.97% in the ipragliflozin group and -0.31% in the placebo group, with an adjusted between-group difference of -0.60% (P<0.001). Compared to placebo, FPG and body weight also decreased significantly (both P<0.001) from baseline after treatment in the ipragliflozin group, with between-group differences of -21.4 mg/dL and -1.53 kg, respectively. Decreased weight was the most common TEAE in the ipragliflozin group (7.0%); there were no reports of genital and urinary tract infection.
CONCLUSION
Ipragliflozin treatment in addition to metformin led to significant improvement in glycemic outcomes and reduction in body weight in Korean patients with type 2 diabetes mellitus, compared with metformin treatment alone; the safety profile was comparable in both groups.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim DJ. The epidemiology of diabetes in Korea. Diabetes Metab J. 2011; 35:303–308. PMID: 21977448.
Article2. Korean Diabetes Association. Korean diabetes fact sheet 2015. Seoul: Korean Diabetes Association;2015.3. Kim DJ, Song KE, Park JW, Cho HK, Lee KW, Huh KB. Clinical characteristics of Korean type 2 diabetic patients in 2005. Diabetes Res Clin Pract. 2007; 77(Suppl 1):S252–S257. PMID: 17459510.
Article4. Korean Diabetes Association. Treatment guideline for diabetes. 5th ed. Seoul: Korean Diabetes Association;2015.5. Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. β-Cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014; 37:1751–1758. PMID: 24812433.
Article6. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009; 301:2129–2140. PMID: 19470990.7. Abdullah N, Attia J, Oldmeadow C, Scott RJ, Holliday EG. The architecture of risk for type 2 diabetes: understanding Asia in the context of global findings. Int J Endocrinol. 2014; 2014:593982. PMID: 24744783.
Article8. Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol. 2001; 280:F10–F18. PMID: 11133510.
Article9. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015; 38:140–149. PMID: 25538310.
Article10. Kashiwagi A, Kazuta K, Goto K, Yoshida S, Ueyama E, Utsuno A. Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2015; 17:304–308. PMID: 24919820.11. Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J Diabetes Investig. 2016; 7:366–373.
Article12. Kashiwagi A, Kazuta K, Takinami Y, Yoshida S, Utsuno A, Nagase I. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int. 2015; 6:8–18.
Article13. Fonseca VA, Ferrannini E, Wilding JP, Wilpshaar W, Dhanjal P, Ball G, Klasen S. Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013; 27:268–273. PMID: 23276620.
Article14. Kashiwagi A, Kazuta K, Yoshida S, Nagase I. Randomized, placebo-controlled, double-blind glycemic control trial of novel sodium-dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig. 2014; 5:382–391.
Article15. Wilding JP, Ferrannini E, Fonseca VA, Wilpshaar W, Dhanjal P, Houzer A. Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab. 2013; 15:403–409. PMID: 23163880.
Article16. Kurosaki E, Ogasawara H. Ipragliflozin and other sodium-glucose cotransporter-2 (SGLT2) inhibitors in the treatment of type 2 diabetes: preclinical and clinical data. Pharmacol Ther. 2013; 139:51–59. PMID: 23563279.
Article17. Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014; 8:262–275. PMID: 24602971.
Article18. Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC. EMPA-REG H2H-SU trial investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014; 2:691–700. PMID: 24948511.19. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013; 382:941–950. PMID: 23850055.
Article20. Ji L, Ma J, Li H, Mansfield TA, T'Joen C L, Iqbal N, Ptaszynska A, List JF. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: a randomized, blinded, prospective phase III study. Clin Ther. 2014; 36:84–100.e9. PMID: 24378206.
Article21. Kaku K, Maegawa H, Tanizawa Y, Kiyosue A, Ide Y, Tokudome T, Hoshino Y, Yang J, Langkilde AM. Dapagliflozin as monotherapy or combination therapy in Japanese patients with type 2 diabetes: an open-label study. Diabetes Ther. 2014; 5:415–433. PMID: 25341477.
Article22. Yoon YS, Choi HS, Kim JK, Kim YI, Oh SW. Differences in the associations of anthropometric measures with insulin resistance and type 2 diabetes mellitus between Korean and US populations: comparisons of representative nationwide sample data. Obes Res Clin Pract. 2016; 10:642–651. PMID: 26750428.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial (Diabetes Metab J 2019;43;276-86)
- Recent Perspective of Metformin
- A Review on Efficacy and Safety of SGLT2 Inhibitors as Add-on Therapy with Metformin
- The Effect of Rosiglitazone and Metformin Therapy, as an Initial Therapy, in Patients with Type 2 Diabetes Mellitus
- Monotherapy in Type 2 Diabetes Mellitus Patients 2017: A Position Statement of the Korean Diabetes Association