Yonsei Med J.
2016 Jul;57(4):942-949. 10.3349/ymj.2016.57.4.942.
AST-120 Improves Microvascular Endothelial Dysfunction in End-Stage Renal Disease Patients Receiving Hemodialysis
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Ewha Womans University, Seoul, Korea. kbchoi@ewha.ac.kr
- KMID: 2374127
- DOI: http://doi.org/10.3349/ymj.2016.57.4.942
Abstract
- PURPOSE
Endothelial dysfunction (ED) is a pivotal phenomenon in the development of cardiovascular disease (CVD) in patients receiving hemodialysis (HD). Indoxyl sulfate (IS) is a known uremic toxin that induces ED in patients with chronic kidney disease. The aim of this study was to investigate whether AST-120, an absorbent of IS, improves microvascular or macrovascular ED in HD patients.
MATERIALS AND METHODS
We conducted a prospective, case-controlled trial. Fourteen patients each were enrolled in respective AST-120 and control groups. The subjects in the AST-120 group were treated with AST-120 (6 g/day) for 6 months. Microvascular function was assessed by laser Doppler flowmetry using iontophoresis of acetylcholine (Ach) and sodium nitroprusside (SNP) at baseline and again at 3 and 6 months. Carotid arterial intima-media thickness (cIMT) and flow-mediated vasodilation were measured at baseline and 6 months. The Wilcoxon rank test was used to compare values before and after AST-120 treatment.
RESULTS
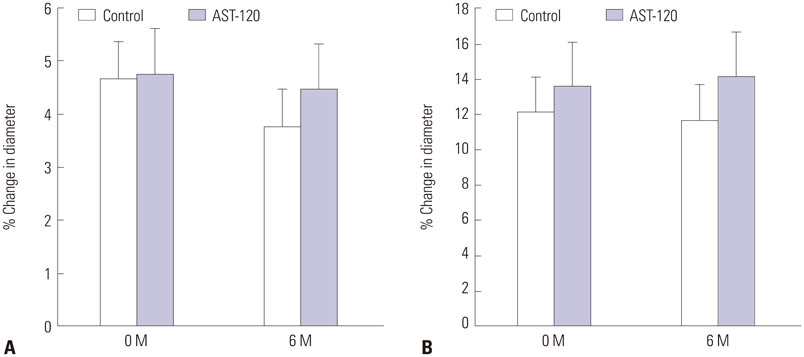
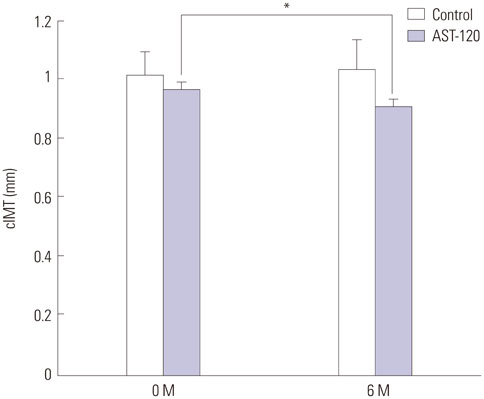
Ach-induced iontophoresis (endothelium-dependent response) was dramatically ameliorated at 3 months and 6 months in the AST-120 group. SNP-induced response showed delayed improvement only at 6 months in the AST-120 group. The IS level was decreased at 3 months in the AST-120 group, but remained stable thereafter. cIMT was significantly reduced after AST-120 treatment. No significant complications in patients taking AST-120 were reported.
CONCLUSION
AST-120 ameliorated microvascular ED and cIMT in HD patients. A randomized study including a larger population will be required to establish a definitive role of AST-120 as a preventive medication for CVD in HD patients.
Keyword
MeSH Terms
-
Acetylcholine
Adult
Carbon/*therapeutic use
Cardiovascular Diseases/etiology/*prevention & control
Carotid Intima-Media Thickness
Endothelium, Vascular/*physiopathology
Female
Humans
Iontophoresis
Kidney Failure, Chronic/complications/*physiopathology/*therapy
Laser-Doppler Flowmetry
Male
Microcirculation/physiology
Middle Aged
Nitroprusside
Oxides/*therapeutic use
Prospective Studies
*Renal Dialysis
Young Adult
Acetylcholine
Carbon
Nitroprusside
Oxides
Figure
Reference
-
1. Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999; 10:1606–1615.
Article2. Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999; 55:648–658.
Article3. Hogas SM, Voroneanu L, Serban DN, Segall L, Hogas MM, Serban IL, et al. Methods and potential biomarkers for the evaluation of endothelial dysfunction in chronic kidney disease: a critical approach. J Am Soc Hypertens. 2010; 4:116–127.
Article4. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012; 2012:918267.
Article5. Corrado E, Rizzo M, Coppola G, Muratori I, Carella M, Novo S. Endothelial dysfunction and carotid lesions are strong predictors of clinical events in patients with early stages of atherosclerosis: a 24-month follow-up study. Coron Artery Dis. 2008; 19:139–144.
Article6. Kruger A, Stewart J, Sahityani R, O'Riordan E, Thompson C, Adler S, et al. Laser Doppler flowmetry detection of endothelial dysfunction in end-stage renal disease patients: correlation with cardiovascular risk. Kidney Int. 2006; 70:157–164.
Article7. Elherik K, Khan F, McLaren M, Kennedy G, Belch JJ. Circadian variation in vascular tone and endothelial cell function in normal males. Clin Sci (Lond). 2002; 102:547–552.
Article8. Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and reversal of premature endothelial cell senescence and vasculopathy in obesity-induced diabetes by ebselen. Circ Res. 2004; 94:377–384.
Article9. Aoyama I, Niwa T. An oral adsorbent ameliorates renal overload of indoxyl sulfate and progression of renal failure in diabetic rats. Am J Kidney Dis. 2001; 37:1 Suppl 2. S7–S12.
Article10. Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, et al. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004; 65:442–451.
Article11. Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009; 4:1551–1558.
Article12. Yu M, Kim YJ, Kang DH. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011; 6:30–39.
Article13. Celermajer DS, Sorensen K, Ryalls M, Robinson J, Thomas O, Leonard JV, et al. Impaired endothelial function occurs in the systemic arteries of children with homozygous homocystinuria but not in their heterozygous parents. J Am Coll Cardiol. 1993; 22:854–858.
Article14. Cupisti A, Rossi M, Placidi S, Fabbri A, Morelli E, Vagheggini G, et al. Responses of the skin microcirculation to acetylcholine in patients with essential hypertension and in normotensive patients with chronic renal failure. Nephron. 2000; 85:114–119.
Article15. Niwayama J, Sanaka T. Development of a new method for monitoring blood purification: the blood flow analysis of the head and foot by laser Doppler blood flowmeter during hemodialysis. Hemodial Int. 2005; 9:56–62.
Article16. Nilsson GE, Tenland T, Oberg PA. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans Biomed Eng. 1980; 27:597–604.
Article17. Tenland T, Salerud EG, Nilsson GE, Oberg PA. Spatial and temporal variations in human skin blood flow. Int J Microcirc Clin Exp. 1983; 2:81–90.18. Morris SJ, Shore AC, Tooke JE. Responses of the skin microcirculation to acetylcholine and sodium nitroprusside in patients with NIDDM. Diabetologia. 1995; 38:1337–1344.
Article19. Davis KR, Ponnampalam J, Hayman R, Baker PN, Arulkumaran S, Donnelly R. Microvascular vasodilator response to acetylcholine is increased in women with pre-eclampsia. BJOG. 2001; 108:610–614.
Article20. Schabauer AM, Rooke TW. Cutaneous laser Doppler flowmetry: applications and findings. Mayo Clin Proc. 1994; 69:564–574.
Article21. Silverman DG, Jotkowitz AB, Freemer M, Gutter V, O'Connor TZ, Braverman IM. Peripheral assessment of phenylephrine-induced vasoconstriction by laser Doppler flowmetry and its potential relevance to homeostatic mechanisms. Circulation. 1994; 90:23–26.
Article22. Yanase T, Nasu S, Mukuta Y, Shimizu Y, Nishihara T, Okabe T, et al. Evaluation of a new carotid intima-media thickness measurement by B-mode ultrasonography using an innovative measurement software, intimascope. Am J Hypertens. 2006; 19:1206–1212.
Article23. Owada A, Nakao M, Koike J, Ujiie K, Tomita K, Shiigai T. Effects of oral adsorbent AST-120 on the progression of chronic renal failure: a randomized controlled study. Kidney Int Suppl. 1997; 63:S188–S190.24. Aoyama I, Miyazaki T, Niwa T. Preventive effects of an oral sorbent on nephropathy in rats. Miner Electrolyte Metab. 1999; 25:365–372.
Article25. Namikoshi T, Tomita N, Satoh M, Sakuta T, Kuwabara A, Kobayashi S, et al. Oral adsorbent AST-120 ameliorates endothelial dysfunction independent of renal function in rats with subtotal nephrectomy. Hypertens Res. 2009; 32:194–200.
Article26. Yamamoto S, Zuo Y, Ma J, Yancey PG, Hunley TE, Motojima M, et al. Oral activated charcoal adsorbent (AST-120) ameliorates extent and instability of atherosclerosis accelerated by kidney disease in apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2011; 26:2491–2497.
Article27. Lee CT, Hsu CY, Tain YL, Ng HY, Cheng BC, Yang CC, et al. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014; 37:76–83.
Article28. Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006; 27:503–508.
Article29. Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010; 2010:453892.
Article30. Witting PK, Rayner BS, Wu BJ, Ellis NA, Stocker R. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell Physiol Biochem. 2007; 20:255–268.
Article31. Ozkor MA, Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiol Res Pract. 2011; 2011:156146.
Article32. Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest. 1997; 100:2793–2799.
Article33. Coats P, Johnston F, MacDonald J, McMurray JJ, Hillier C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation. 2001; 103:1702–1708.34. Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005; 111:796–803.
Article35. Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol. 2005; 563(Pt 3):965–973.
Article36. Durand S, Tartas M, Bouyé P, Koïtka A, Saumet JL, Abraham P. Prostaglandins participate in the late phase of the vascular response to acetylcholine iontophoresis in humans. J Physiol. 2004; 561(Pt 3):811–819.
Article37. Nakamura T, Kawagoe Y, Matsuda T, Ueda Y, Shimada N, Ebihara I, et al. Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press Res. 2004; 27:121–126.
Article38. Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996; 496(Pt 2):531–542.
Article39. Farkas K, Kolossváry E, Járai Z, Nemcsik J, Farsang C. Non-invasive assessment of microvascular endothelial function by laser Doppler flowmetry in patients with essential hypertension. Atherosclerosis. 2004; 173:97–102.
Article40. Brown H, Moppett IK, Mahajan RP. Transient hyperaemic response to assess vascular reactivity of skin: effect of locally iontophoresed acetylcholine, bradykinin, epinephrine and phenylephrine. Br J Anaesth. 2003; 90:446–451.
Article41. Fredriksson I, Larsson M, Strömberg T. Measurement depth and volume in laser Doppler flowmetry. Microvasc Res. 2009; 78:4–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Crohn's Disease in a Patient Undergoing Hemodialysis Caused by IgA Nephropathy
- Affecting Factors on Endothelial Dysfunction in Diabetic End Stage Renal Disease Patients on Hemodialysis
- Phenomenology on the Hemodialysis Experience of Patients with End-Stage Renal Disease
- Acquired Cystic Kidney Disease in Patients Undergoing Long-term Hemodialysis Treatment
- Survey on kidney transplantation awareness among hemodialysis patients at a single center in Japan