Ann Lab Med.
2016 May;36(3):271-274. 10.3343/alm.2016.36.3.271.
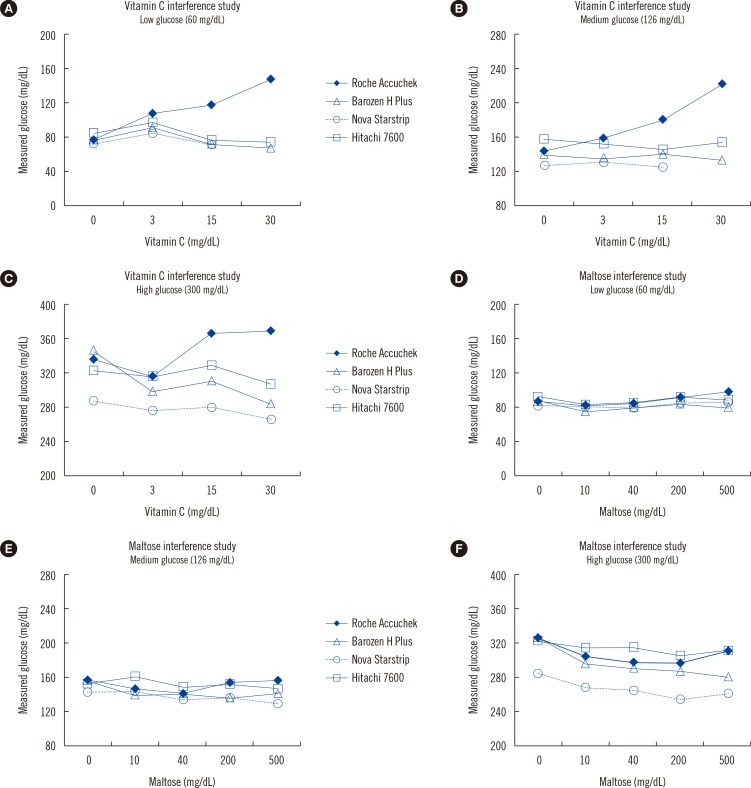
Influence of Vitamin C and Maltose on the Accuracy of Three Models of Glucose Meters
- Affiliations
-
- 1Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. comforter6@yuhs.ac
- 2Department of Laboratory Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2373541
- DOI: http://doi.org/10.3343/alm.2016.36.3.271
Abstract
- No abstract available.
MeSH Terms
Figure
Reference
-
1. Yoo EH, Lee SY. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel). 2010; 10:4558–4576. PMID: 22399892.
Article2. Kim SK, Hahm JR, Kim HS, Kim S, Jung TS, Jung JH, et al. Spurious elevation of glucose concentration during administration of high dose of ascorbic acid in a patient with type 2 diabetes on hemodialysis. Yonsei Med J. 2013; 54:1289–1292. PMID: 23918584.
Article3. Riley SG, Chess J, Donovan KL, Williams JD. Spurious hyperglycaemia and icodextrin in peritoneal dialysis fluid. BMJ. 2003; 327:608–609. PMID: 12969932.
Article4. Moon HW, Kim JY, Kang ES, Chung WS. Interference with the measurement of blood glucose in different systems after intravenous high dose ascorbic acid supplement. Korean J Lab Med. 2005; 25:294–299.5. Heller A, Feldman B. Electrochemical glucose sensors and their applications in diabetes management. Chem Rev. 2008; 108:2482–2505. PMID: 18465900.
Article6. Kim YB, Seo JY, Lee SY, Park HD. Performance evaluation of glucometer Barozen H based on ISO 15197 standards. Lab Med Online. 2015; 5:6–14.
Article7. Tang Z, Du X, Louie RF, Kost GJ. Effects of drugs on glucose measurements with handheld glucose meters and a portable glucose analyzer. Am J Clin Pathol. 2000; 113:75–86. PMID: 10631860.
Article8. Ng WY, Tiong CC, Jacob E. Maltose interference-free test strips for blood glucose testing at point-of-care: a laboratory performance evaluation. Diabetes Technol Ther. 2010; 12:889–893. PMID: 20879959.
Article9. International Organization for Standardization. In vitro diagnostic test systems: Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197. Geneva: ISO;2013(E).
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Self-Monitoring Blood Glucose Meter: Is Your Glucose Meter Accurate?
- Effects of 5 % Maltose in Lactated Ringers Solution on Blood. Glucose and pH during Operation
- Application of Six Sigma Metrics to Improve Quality Control for Point-of-care Glucose Testing
- Evaluation of ACCU-CHEK(R) Inform II Blood Glucose Meter and ACCU-CHEK(R) Performa Strip
- Blood glucose analysis in patients on continuous ambulatory peritoneal dialysis with Icodextrin