Ewha Med J.
2020 Jul;43(3):43-48. 10.12771/emj.2020.43.3.43.
Application of Six Sigma Metrics to Improve Quality Control for Point-of-care Glucose Testing
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2St. Mary’s Hospital, Pyeongtaek, Korea
- KMID: 2505143
- DOI: http://doi.org/10.12771/emj.2020.43.3.43
Abstract
Objectives
Six sigma is a quality management system for the assessment of precisionand accuracy. We aim to apply the six sigma rule to quality control (QC) of point-of-care(POC) glucose meters in a tertiary hospital.
Methods
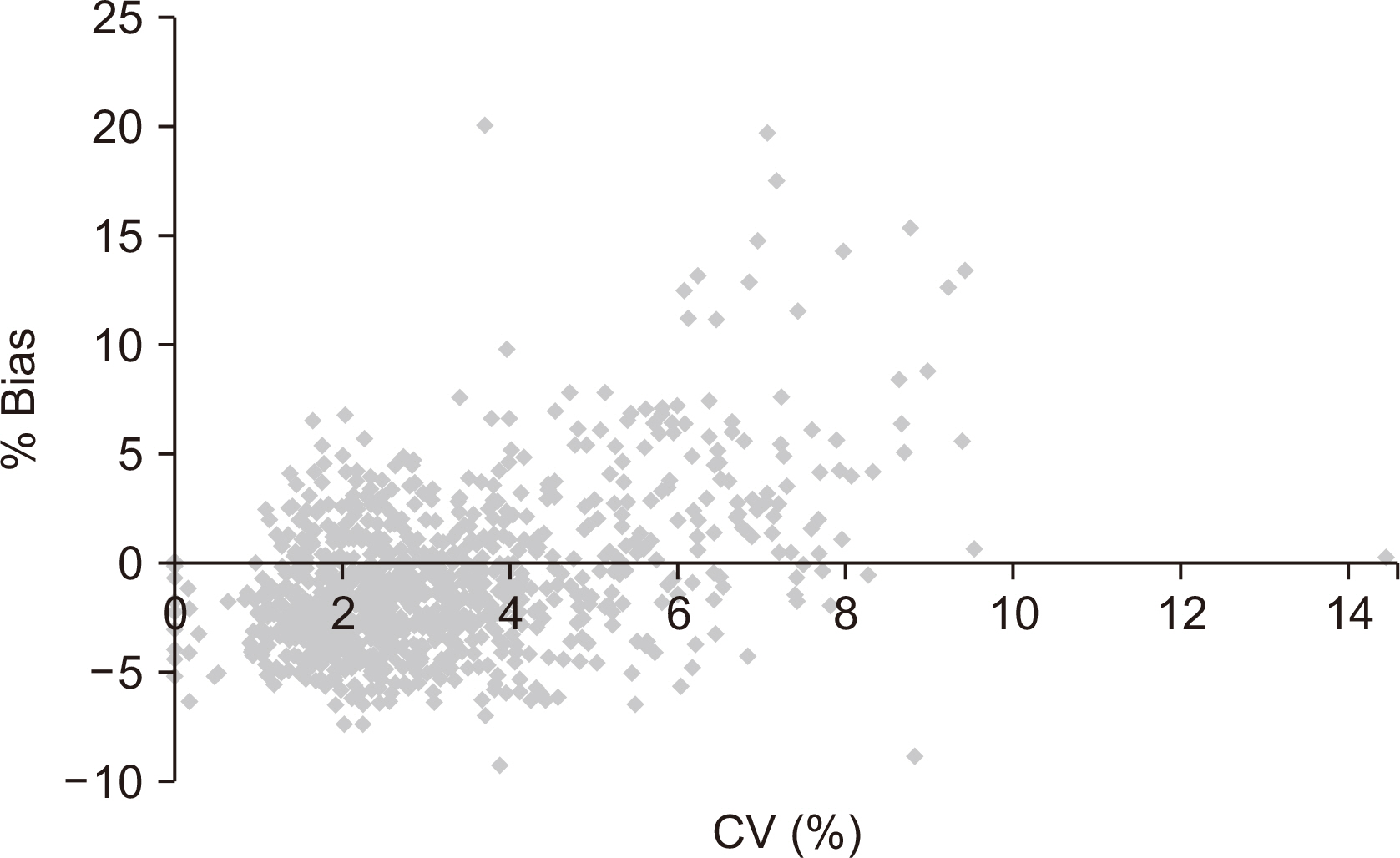
Thirty POC glucose meters installed at Ewha Womans University MokdongHospital were monitored between January 2013 and March 2014. The QC data fromthe POC glucose meters at low and high levels were collected. The monthly mean, standarddeviation, bias, coefficient of variation, and mean sigma metrics were calculated.The correlation between accuracy and precision was assessed based on the percentagebias and coefficient of variation. Comprehensive instructions on the QC and maintenanceof the devices were provided in the departments with poor sigma scores. Afollow-up assessment was performed after the intervention.
Results
The mean sigma values for the low and high controls were 3.29 and 3.71, respectively.At the low and high controls, 36.6% and 10% of the glucose meters showeda sigma value <3. The causes of low sigma values included the use of expired controlmaterials, prolonged air exposure of the sample strip, lack of user training, and errors indevice maintenance. On follow-up monitoring for 3 months following QC intervention,23.3% (low control) and 6.6% (high control) of the glucose meters scored a sigma value<3, indicating improved QC.
Conclusion
Sigma metrics-based QC can successfully improve accuracy and precisionof POC glucose meters in an objective and quantitative manner and can be usedfor follow up after QC intervention.
Figure
Reference
-
1. Gras JM, Philippe M. 2007; Application of the Six Sigma concept in clinical laboratories: a review. Clin Chem Lab Med. 45:789–796. DOI: 10.1515/CCLM.2007.135. PMID: 17579533.
Article2. Westgard JO, Westgard SA. 2006; The quality of laboratory testing today: an assessment of sigma metrics for analytic quality using performance data from proficiency testing surveys and the CLIA criteria for acceptable performance. Am J Clin Pathol. 125:343–354. DOI: 10.1309/V50H4FRVVWX12C79. PMID: 16613337.3. Nanda SK, Ray L. 2013; Quantitative application of sigma metrics in medical biochemistry. J Clin Diagn Res. 7:2689–2691. DOI: 10.7860/JCDR/2013/7292.3700. PMID: 24551613. PMCID: PMC3919274.
Article4. Kjome RL, Granas AG, Nerhus K, Sandberg S. 2010; Quality assessment of patients’ self-monitoring of blood glucose in community pharmacies. Pharm Pract (Granada). 8:62–69. DOI: 10.4321/S1886-36552010000100008. PMID: 25152795.
Article5. Perich C, Minchinela J, Ricos C, Fernandez-Calle P, Alvarez V, Domenech MV, et al. 2015; Biological variation database: structure and criteria used for generation and update. Clin Chem Lab Med. 53:299–305. DOI: 10.1515/cclm-2014-0739. PMID: 25415636.
Article6. Westgard S. 2013; Prioritizing risk analysis quality control plans based on Sigma-metrics. Clin Lab Med. 33:41–53. DOI: 10.1016/j.cll.2012.11.008. PMID: 23331728.
Article7. Gill JP, Shephard MD. 2010; The conduct of quality control and quality assurance testing for PoCT outside the laboratory. Clin Biochem Rev. 31:85–88. PMID: 24150510. PMCID: PMC2924127.8. Burtis CA, Ashwood ER, Bruns DE. 2012. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. Elservier;St Louis:9. Liu LF, Lee S, Chia PF, Chi SC, Yin YC. 2012; Exploring the association between nurse workload and nurse-sensitive patient safety outcome indicators. J Nurs Res. 20:300–309. DOI: 10.1097/jnr.0b013e3182736363. PMID: 23154441.
Article10. Magalhaes AM, Costa DG, Riboldi CO, Mergen T, Barbosa AD, Moura GM. 2017; Association between workload of the nursing staff and patient safety outcomes. Rev Esc Enferm USP. 51:e03255. DOI: 10.1590/s1980-220x2016021203255. PMID: 29211232.
Article11. Westgard JO, Stein B. 1997; Automated selection of statistical quality-control procedures to assure meeting clinical or analytical quality requirements. Clin Chem. 43:400–403. DOI: 10.1093/clinchem/43.2.400. PMID: 9023147.
Article12. Hens K, Berth M, Armbruster D, Westgard S. 2014; Sigma metrics used to assess analytical quality of clinical chemistry assays: importance of the allowable total error (TEa) target. Clin Chem Lab Med. 52:973–980. DOI: 10.1515/cclm-2013-1090. PMID: 24615486.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluating the Performance of an Automated Chemistry Analyzer Using Sigma Metrics
- Evaluation of Fixed Quality Control Range of Bayer Rapidpoint 400 Blood Gas and Electrolyte Analyzer with Six Sigma Metrics
- Report on the External Quality Assessment Scheme for Blood Gas (Central Laboratory and Point-of-Care Testing) and Glucose (Point-of-Care Testing) Analysis in Korea (2016–2017)
- Evaluation of the Performance of a Micromethod for Measuring Urinary Iodine by Using Six Sigma Quality Metrics
- Development of a Systematic Quality Control Program for Point-of-Care Glucose Testing