Korean J Gastroenterol.
2015 Apr;65(4):205-214. 10.4166/kjg.2015.65.4.205.
Gastrointestinal Bleeding with Dabigatran, a Comparative Study with Warfarin: A Multicenter Experience
- Affiliations
-
- 1Section of Gastroenterology and Hepatology, Georgia Regents University, Augusta, GA, USA. muhammedsherid@gmail.com
- 2Division of Gastroenterology, Department of Internal Medicine, Saint Francis Hospital, Evanston, IL, USA.
- 3Department of Medicine, Northwestern Memorial Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
- 4Division of Gastroenterology, Department of Internal Medicine, Trinitas Regional Medical Center, Seton Hall University, School of Health and Medicine Sciences, Elizabeth, NJ, USA.
- KMID: 2373211
- DOI: http://doi.org/10.4166/kjg.2015.65.4.205
Abstract
- BACKGROUND/AIMS
The risk of gastrointestinal (GI) bleeding with dabigatran when compared to warfarin has been controversial in the literature. The aim of our study was to assess this risk with the use of dabigatran.
METHODS
We examined the medical records of patients who were started on dabigatran or warfarin from October 2010 to October 2012. The study was conducted in two hospitals.
RESULTS
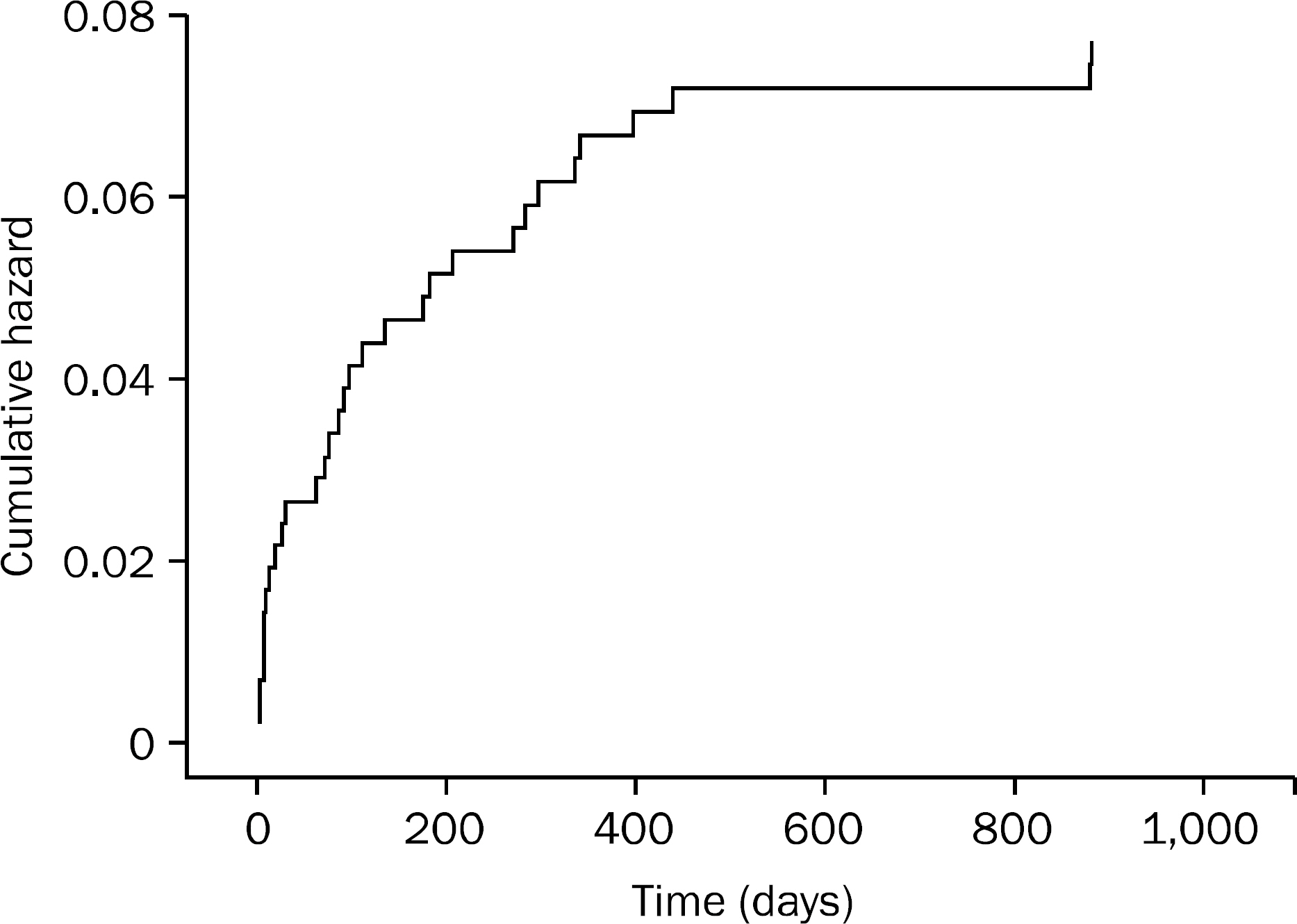
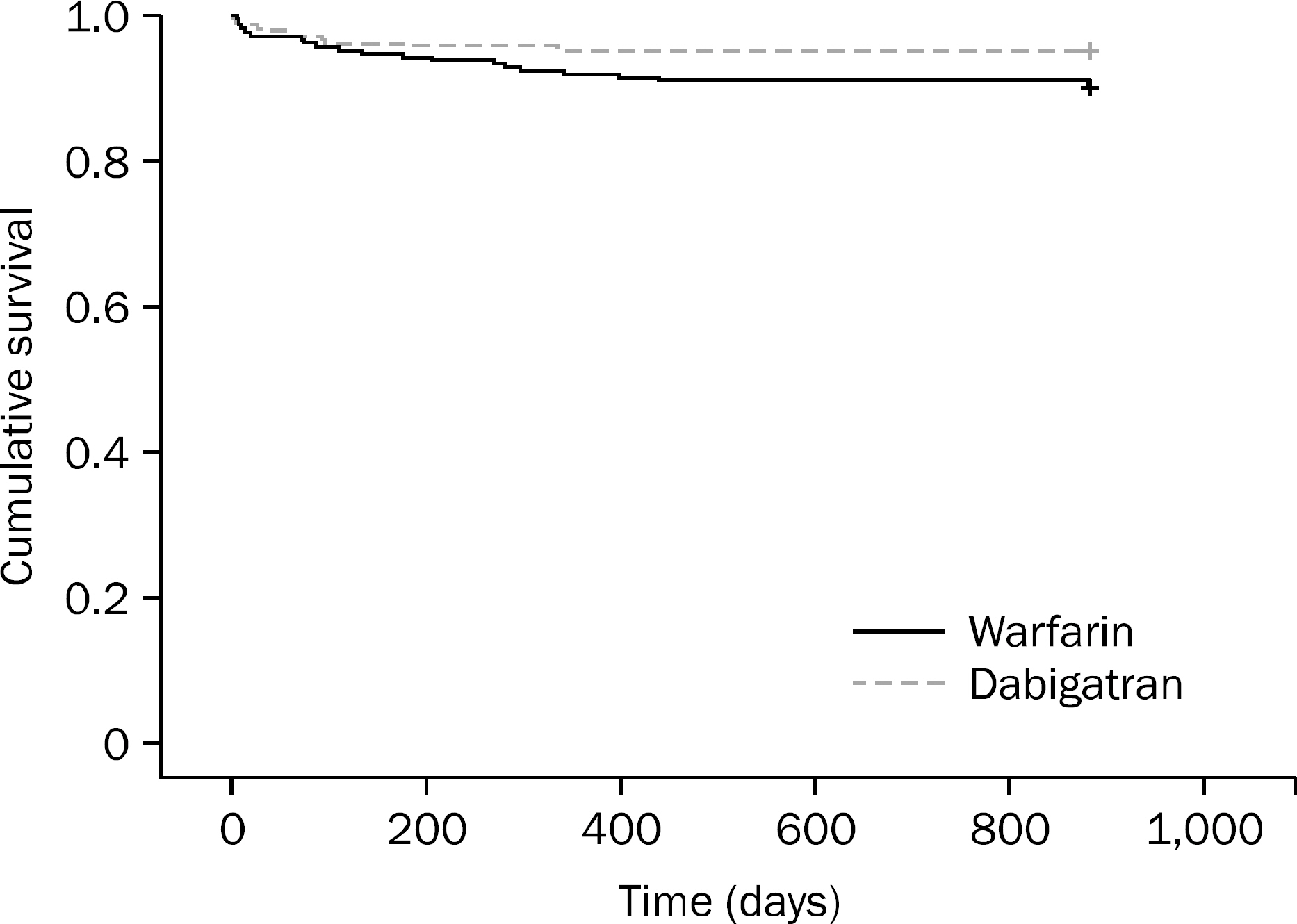
A total of 417 patients were included (208 dabigatran vs. 209 warfarin). GI bleeding occurred in 10 patients (4.8%) in the dabigatran group compared to 21 patients (10.1%) in the warfarin group (p=0.0375). Multivariate analysis showed that patients who were on dabigatran for < or =100 days had a higher incidence of GI bleeding than those who were on it for >100 days (p=0.0007). The odds of GI bleeding in patients who were on dabigatran for < or =100 days was 8.2 times higher compared to those who were on the drug for >100 days. The incidence of GI bleeding in patients >65 years old was higher than in those <65 years old (p=0.0453, OR=3). History of previous GI bleeding was another risk factor for GI bleeding in the dabigatran group (p=0.036, OR=6.3). The lower GI tract was the most common site for GI bleeding in the dabigatran group (80.0% vs. 38.1%, p=0.014).
CONCLUSIONS
The risk of GI bleeding was lower with dabigatran. The risk factors for GI bleeding with dabigtran were the first 100 days, age >65 years, and a history of previous GI bleeding.
Keyword
MeSH Terms
-
Age Factors
Aged
Aged, 80 and over
Anticoagulants/*adverse effects/therapeutic use
Atrial Fibrillation/drug therapy
Dabigatran/*adverse effects/therapeutic use
Female
Gastrointestinal Hemorrhage/*chemically induced/epidemiology/mortality
Humans
Incidence
Kaplan-Meier Estimate
Male
Middle Aged
Multivariate Analysis
Odds Ratio
Retrospective Studies
Risk Factors
Warfarin/*adverse effects/therapeutic use
Anticoagulants
Dabigatran
Warfarin
Figure
Reference
-
References
1. Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.
Article2. Schulman S, Kearon C, Kakkar AK, et al. RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009; 361:2342–2352.
Article3. Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007; 64:292–303.
Article4. Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008; 47:47–59.5. Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal im-pairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010; 49:259–268.6. Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract. 2010; 64:956–967.
Article7. Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013; 127:634–640.
Article8. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011; 123:2363–2372.9. Larsen TB, Rasmussen LH, Skj⊘th F, et al. Efficacy and safety of dabigatran etexilate and warfarin in “real-world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013; 61:2264–2273.10. Eriksson BI, Dahl OE, Büller HR, et al. BISTRO II Study Group. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005; 3:103–111.
Article11. Connolly SJ, Pogue J, Eikelboom J, et al. ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008; 118:2029–2037.
Article12. White HD, Gruber M, Feyzi J, et al. Comparison of outcomes among patients randomized to warfarin therapy according to anticoagulant control: results from SPORTIF III and V. Arch Intern Med. 2007; 167:239–245.13. Jones M, McEwan P, Morgan CL, Peters JR, Goodfellow J, Currie CJ. Evaluation of the pattern of treatment, level of anticoagulation control, and outcome of treatment with warfarin in patients with non-valvar atrial fibrillation: a record linkage study in a large British population. Heart. 2005; 91:472–477.
Article14. Wallentin L, Yusuf S, Ezekowitz MD, et al. RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at dif-ferent levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010; 376:975–983.
Article15. Samsa GP, Matchar DB, Goldstein LB, et al. Quality of anticoagulation management among patients with atrial fibrillation: results of a review of medical records from 2 communities. Arch Intern Med. 2000; 160:967–973.16. Sarawate C, Sikirica MV, Willey VJ, Bullano MF, Hauch O. Monitoring anticoagulation in atrial fibrillation. J Thromb Thrombolysis. 2006; 21:191–198.
Article17. McBride D, Brüggenjürgen B, Roll S, Willich SN. Anticoagulation treatment for the reduction of stroke in atrial fibrillation: a cohort study to examine the gap between guidelines and routine medical practice. J Thromb Thrombolysis. 2007; 24:65–72.
Article18. Southworth MR, Reichman ME, Unger EF. Dabigatran and post-marketing reports of bleeding. N Engl J Med. 2013; 368:1272–1274.
Article19. Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators. Newly identified events in the RE-LY trial. N Engl J Med. 2010; 363:1875–1876.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Uninterrupted Dabigatran versus Warfarin for Ablation in Atrial Fibrillation
- Risk Factors of Gastrointestinal Bleeding in Patients Receiving New Oral Anticoagulants
- Drug Utilization of Oral Anticoagulants in Patients with Non-valvular Atrial Fibrillation in the Elderly
- Clinical outcomes of direct-acting oral anticoagulants compared to warfarin in patients with non-valvular atrial fibrillation
- Real-world Data and Recommended Dosage of Non-vitamin K Oral Anticoagulants for Korean Patients