J Gastric Cancer.
2016 Mar;16(1):58-62. 10.5230/jgc.2016.16.1.58.
Gastrointestinal Stromal Tumor of the Stomach Presenting as Multilobular with Diffuse Calcifications
- Affiliations
-
- 1Department of Internal Medicine, Eulji University Hospital, Daejeon, Korea.
- 2Department of Surgery, Eulji University Hospital, Daejeon, Korea. mslee01@eulji.ac.kr
- 3Department of Radiology, Eulji University Hospital, Daejeon, Korea.
- 4Department of Pathology, Eulji University Hospital, Daejeon, Korea.
- KMID: 2372555
- DOI: http://doi.org/10.5230/jgc.2016.16.1.58
Abstract
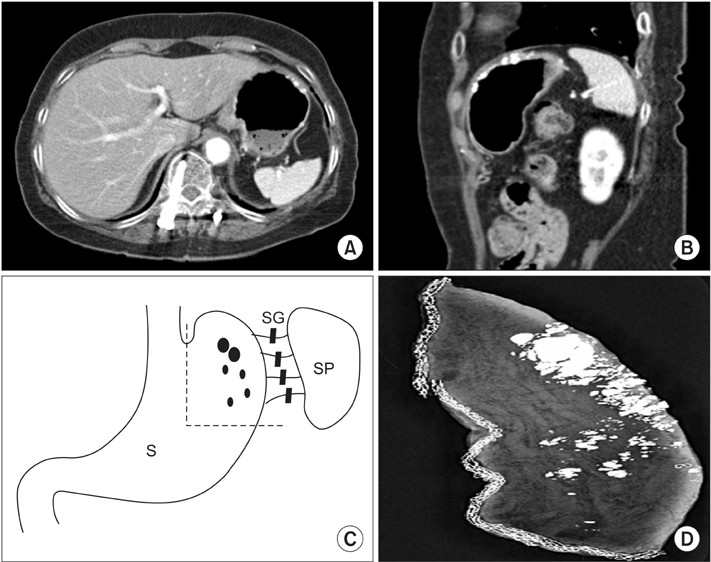
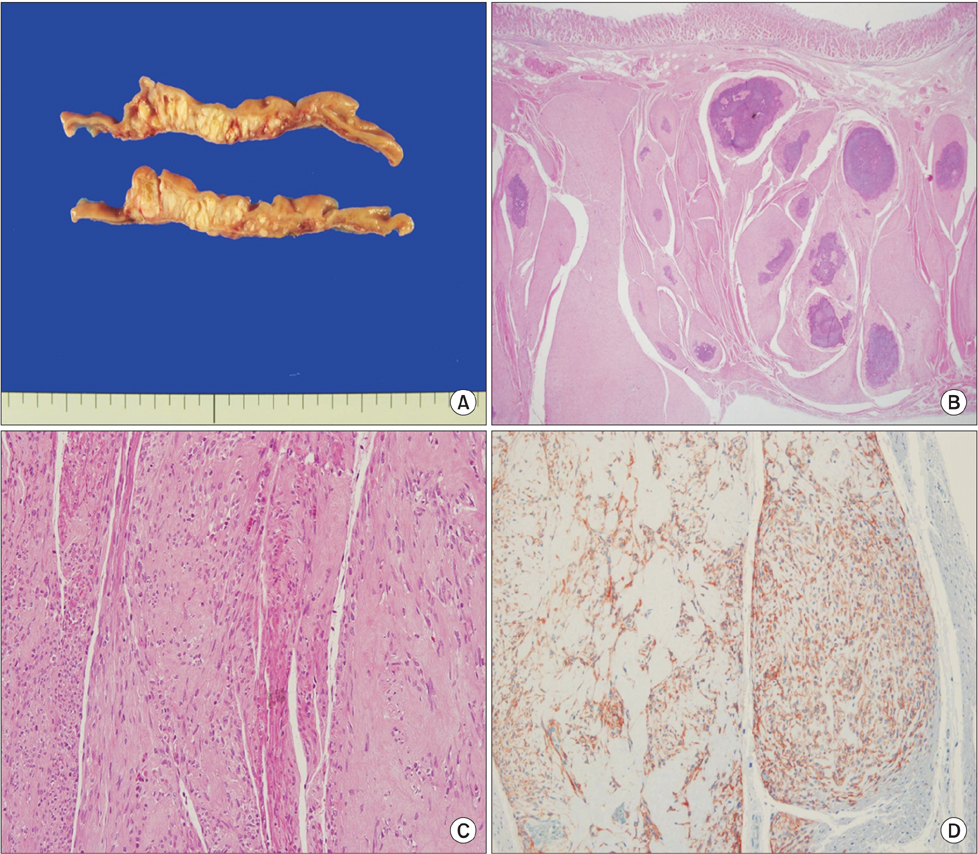
- Gastrointestinal stromal tumors (GISTs) are the most common primary mesenchymal neoplasms of the gastrointestinal tract and usually appear as a well-circumscribed mass. However, it may be difficult to confirm the extent of the disease for some GISTs. A 70-year-old asymptomatic female presented for a regular physical exam. An esophagogastroduodenoscopy showed a 2.0 cm protruding mass on the gastric fundus. Endoscopic ultrasound revealed an ill-defined heterogenous hypoechoic lesion (3.0×1.5 cm). A computed tomography (CT) scan demonstrated a 4.5 cm multifocal calcified mass at the gastric body as well as at the gastric fundus. Laparoscopic gastric wedge resection was performed according to the extent of multifocal calcifications that are shown on the CT. Intraoperative specimen mammography and intraoperative biopsy might be helpful to obtain a tumor-free margin. Final pathologic diagnosis was an intermediate risk GIST in multilobular form. In patients with diffuse multifocal calcifications in the stomach, the possibility of GIST should be considered.
Keyword
MeSH Terms
Figure
Reference
-
1. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000; 15:1293–1301.2. Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010; 25:853–862.3. Agaimy A, Wünsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically reevaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006; 391:322–329.4. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003; 23:283–304.5. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.6. Chourmouzi D, Sinakos E, Papalavrentios L, Akriviadis E, Drevelegas A. Gastrointestinal stromal tumors: a pictorial review. J Gastrointestin Liver Dis. 2009; 18:379–383.7. Ghahremani GG, Meyers MA, Port RB. Calcified primary tumors of the gastrointestinal tract. Gastrointest Radiol. 1978; 2:331–339.8. Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003; 13:1669–1678.9. Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. "Pediatric-type" gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011; 35:495–504.10. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. GIST consensus meeting panelists. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.11. Rutkowski P, Nowecki ZI, Michej W, Debiec-Rychter M, Woźniak A, Limon J, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol. 2007; 14:2018–2127.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Gastric Calcifying Fibrous Tumor Presenting as a Subepithelial Tumor
- A case of huge Gastrointestinal stromal tumor masquerading as an ovarian malignancy
- Gastrointestinal Stromal Tumor in a Patient Complaining about Less Decreasing Abdominal Obesity
- A Face of Gastrointestinal Stromal Tumor
- A Case of Epithelioid Type Gastric Gastrointestinal Stromal Tumor with Gastrointestinal Bleeding