J Korean Med Sci.
2017 Apr;32(4):666-671. 10.3346/jkms.2017.32.4.666.
Safety of Using Matrix Metalloproteinase Inhibitor in Experimental Glaucoma Filtration Surgery
- Affiliations
-
- 1Department of Ophthalmology, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea. scarpel@hally.or.kr
- 2Department of Ophthalmology, Institute of Ophthalmology and Optometry, Ewha Womans University Mok-Dong Hospital, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 2371454
- DOI: http://doi.org/10.3346/jkms.2017.32.4.666
Abstract
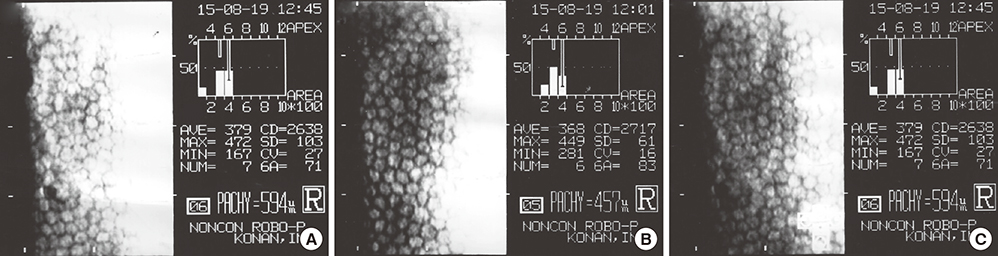
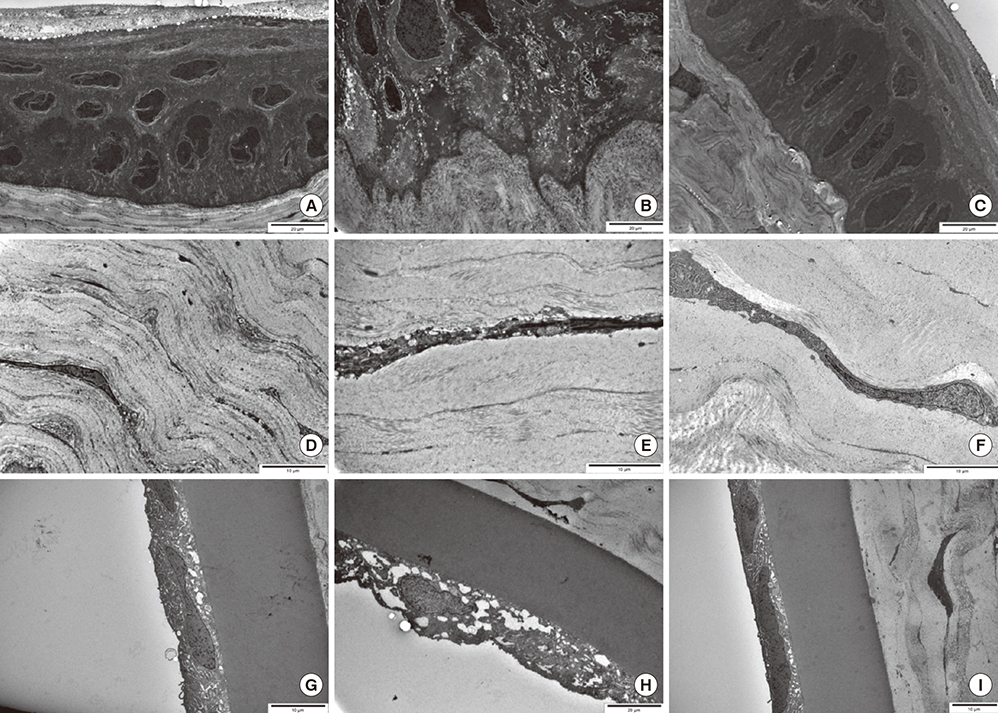
- We evaluated the safety of matrix metalloproteinase (MMP) inhibitor in experimental glaucoma filtration surgery in an animal model. Fifteen New Zealand white rabbits underwent an experimental trabeculectomy and were randomly allocated into 3 groups according to the adjuvant agent: no treatment group (n = 5), 0.02% mitomycin C (MMC) soaking group (n = 5), and MMP inhibitor (ilomastat) subconjunctival injection group (n = 5). Slit lamp examination with Seidel testing, pachymetry, and specular microscopy was performed preoperatively and postoperatively. The conjunctiva and ciliary body toxicity were evaluated with scores according to the pathologic grading systems. Electron microscopy was used to examine the structural changes in cornea, conjunctiva, and ciliary body. In the ilomastat-treated group, there was no statistically significant change in central corneal thickness preoperatively and at 28 days postoperatively (P = 0.655). There were also no significant changes in specular microscopy findings over the duration of the study in the ilomastat-treated group. The conjunctival toxicity score was 1 in the control group, 1.5 in the ilomastat-treated group, and 2 in the MMC-treated group. When assessing ciliary body toxicity scores, the ilomastat-treated group score was 0.5 and the MMC-treated group score was 1.5. Transmission electron microscopy did not show structural changes in the cornea and ciliary body whereas the structural changes were noticed in MMC group. A single subconjunctival injection of MMP inhibitor during the experimental trabeculectomy showed a less toxic affect in the rabbit cornea, conjunctiva, and ciliary body compared to MMC.
Figure
Reference
-
1. Filtering surgery. In : Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G, Shields MB, editors. Shields' Textbook of Glaucoma. 5th ed. Philadelphia, PA: Lippincott Willliams & Wilkins;2005. p. 568–609.2. Jampel HD. Effect of brief exposure to mitomycin C on viability and proliferation of cultured human Tenon’s capsule fibroblasts. Ophthalmology. 1992; 99:1471–1476.3. Smith S, D’Amore PA, Dreyer EB. Comparative toxicity of mitomycin C and 5-fluorouracil in vitro. Am J Ophthalmol. 1994; 118:332–337.4. Nuyts RM, Felten PC, Pels E, Langerhorst CT, Geijssen HC, Grossniklaus HE, Greve EL. Histopathologic effects of mitomycin C after trabeculectomy in human glaucomatous eyes with persistent hypotony. Am J Ophthalmol. 1994; 118:225–237.5. Hau S, Barton K. Corneal complications of glaucoma surgery. Curr Opin Ophthalmol. 2009; 20:131–136.6. Pastor SA, Williams R, Hetherington J, Hoskins HD, Goodman D. Corneal endothelial cell loss following trabeculectomy with mitomycin C. J Glaucoma. 1993; 2:112–113.7. Shima I, Katsuda S, Ueda Y, Takahashi N, Sasaki H. Expression of matrix metalloproteinases in wound healing after glaucoma filtration surgery in rabbits. Ophthalmic Res. 2007; 39:315–324.8. Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2003; 44:1097–1103.9. Martorana GM, Schaefer JL, Levine MA, Lukowski ZL, Min J, Meyers CA, Schultz GS, Sherwood MB. Sequential therapy with saratin, bevacizumab and ilomastat to prolong bleb function following glaucoma filtration surgery in a rabbit model. PLoS One. 2015; 10:e0138054.10. Wong TT, Mead AL, Khaw PT. Prolonged antiscarring effects of ilomastat and MMC after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2005; 46:2018–2022.11. Lim DH, Kim TE, Kee C. Evaluation of adenovirus-mediated down-regulation of connective tissue growth factor on postoperative wound healing after experimental glaucoma surgery. Curr Eye Res. 2016; 41:951–956.12. Polak MB, Valamanesh F, Felt O, Torriglia A, Jeanny JC, Bourges JL, Rat P, Thomas-Doyle A, BenEzra D, Gurny R, et al. Controlled delivery of 5-chlorouracil using poly(ortho esters) in filtering surgery for glaucoma. Invest Ophthalmol Vis Sci. 2008; 49:2993–3003.13. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363:1711–1720.14. Spaeth G, Walt J, Keener J. Evaluation of quality of life for patients with glaucoma. Am J Ophthalmol. 2006; 141:S3–14.15. Suh W, Kee C. The effect of bevacizumab on the outcome of trabeculectomy with 5-Fluorouracil. J Ocul Pharmacol Ther. 2013; 29:646–651.16. Dorr RT. New findings in the pharmacokinetic, metabolic, and drug-resistance aspects of mitomycin C. Semin Oncol. 1988; 15:32–41.17. Parikh CH, Edelhauser HF. Ocular surgical pharmacology: corneal endothelial safety and toxicity. Curr Opin Ophthalmol. 2003; 14:178–185.18. Nishida T, Saika S. Basic science: cornea, sclera, ocular adnexa anatomy, physiology and pathophysiologic responses. In : Krachmer JH, Mannis MJ, Holland E, editors. Cornea. 3rd ed. St. Louis, Mo: Mosby/Elsevier;2011. p. 3–24.19. Zarei R, Zarei M, Fakhraie G, Eslami Y, Moghimi S, Mohammadi M, Abdollahi A. Effect of mitomycin-C augmented trabeculectomy on corneal endothelial cells. J Ophthalmic Vis Res. 2015; 10:257–262.20. Fukuchi T, Hayakawa Y, Hara H, Abe H. Corneal endothelial damage after trabeculectomy with mitomycin C in two patients with glaucoma with cornea guttata. Cornea. 2002; 21:300–304.21. Daniels JT, Cambrey AD, Occleston NL, Garrett Q, Tarnuzzer RW, Schultz GS, Khaw PT. Matrix metalloproteinase inhibition modulates fibroblast-mediated matrix contraction and collagen production in vitro. Invest Ophthalmol Vis Sci. 2003; 44:1104–1110.22. Xia X, Jiang Y, Huang P, Wu Z, Zeng Q, Wen J. Cytotoxic effect of mitomycin C on the nonpigmented epithelium of ciliary body in rabbit eyes. Zhonghua Yan Ke Za Zhi. 1998; 34:190–193.23. Cetinkaya A, Akman A, Take G, Bilezikci B, Akova YA. Ciliary body toxicity of subconjunctival suramin compared with mitomycin-C in the rabbit eye: determining the toxic concentration. Ophthalmic Res. 2009; 41:91–97.24. Kawase K, Matsushita H, Yamamoto T, Kitazawa Y. Mitomycin concentration in rabbit and human ocular tissues after topical administration. Ophthalmology. 1992; 99:203–207.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of the Levels of Trabecular Matrix Metalloproteinase and Inhibitor by Transforming Growth Factor-beta1
- Matrix Metalloproteinases and Their Inhibitors in Gastric Carcinoma
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients
- The Relationship Between the Activity of Matrix Metalloproteinase in the Aqueous Humor and the Development of Primary Open Angle Glaucoma
- Matrix Metalloproteinase-1 and Tissue Inhibitor of Metalloproteinase-1 levels in Exudative Pleural Effusions