J Korean Soc Transplant.
2016 Dec;30(4):165-171. 10.4285/jkstn.2016.30.4.165.
Sirolimus Combination with Tacrolimus in Kidney Transplant Recipients at High Immunological Risk: Observational Results 3 Years after Transplantation
- Affiliations
-
- 1Department of Surgery, Yonsei University College of Medicine, Seoul, Korea. yukim@yuhs.ac
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2364936
- DOI: http://doi.org/10.4285/jkstn.2016.30.4.165
Abstract
- BACKGROUND
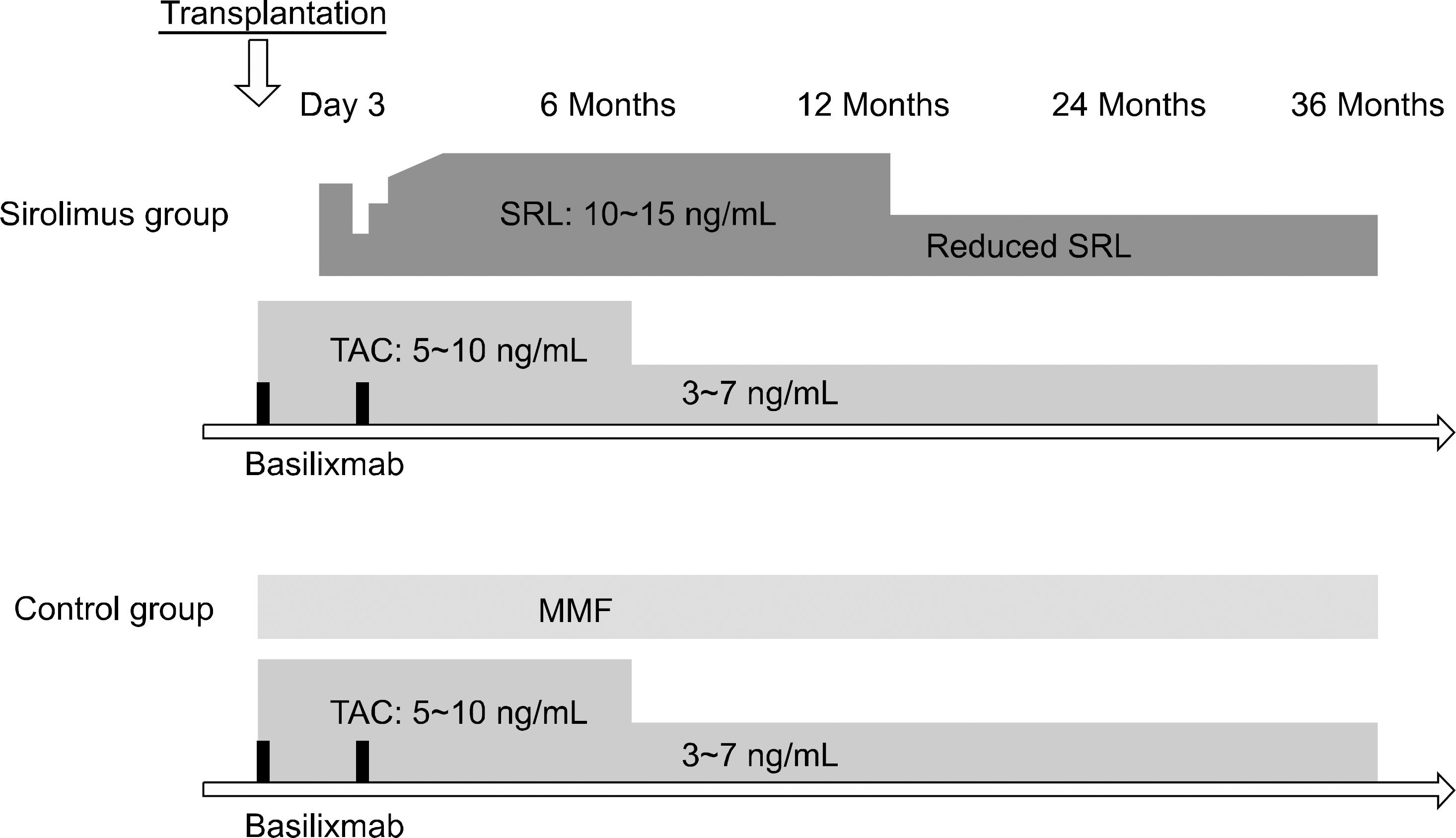
The optimal immunosuppressive strategy for renal transplant recipients at high immunological risk requires clarification. We compared the 3 year outcomes of a sirolimus group (tacrolimus plus sirolimus) to those of a control group (tacrolimus plus mycophenolate mofetil).
METHODS
This observational study was an extension of a prospective pilot study. We assessed acute rejection, glomerular filtration rate, adverse events, graft, and patient survival.
RESULTS
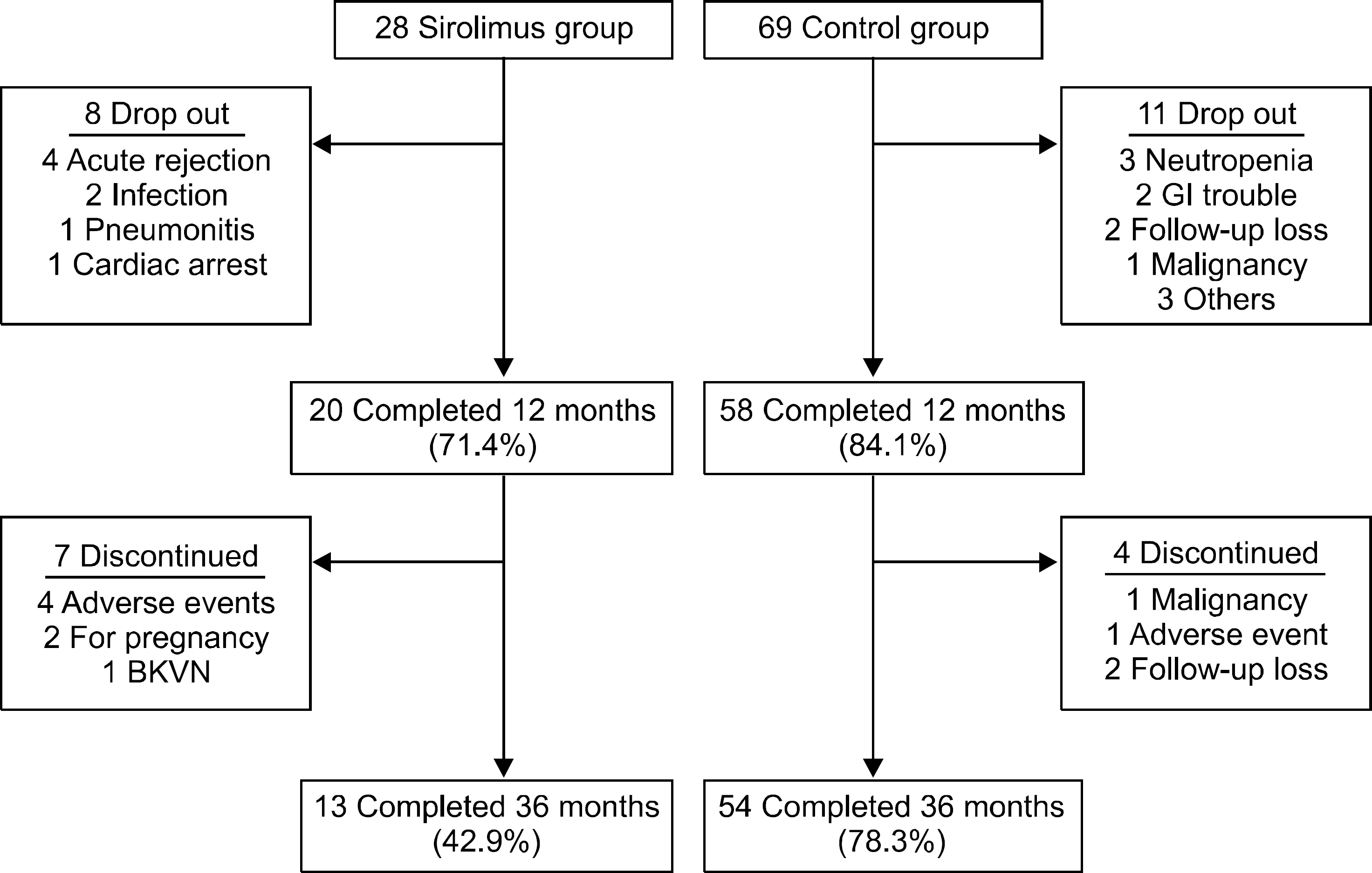
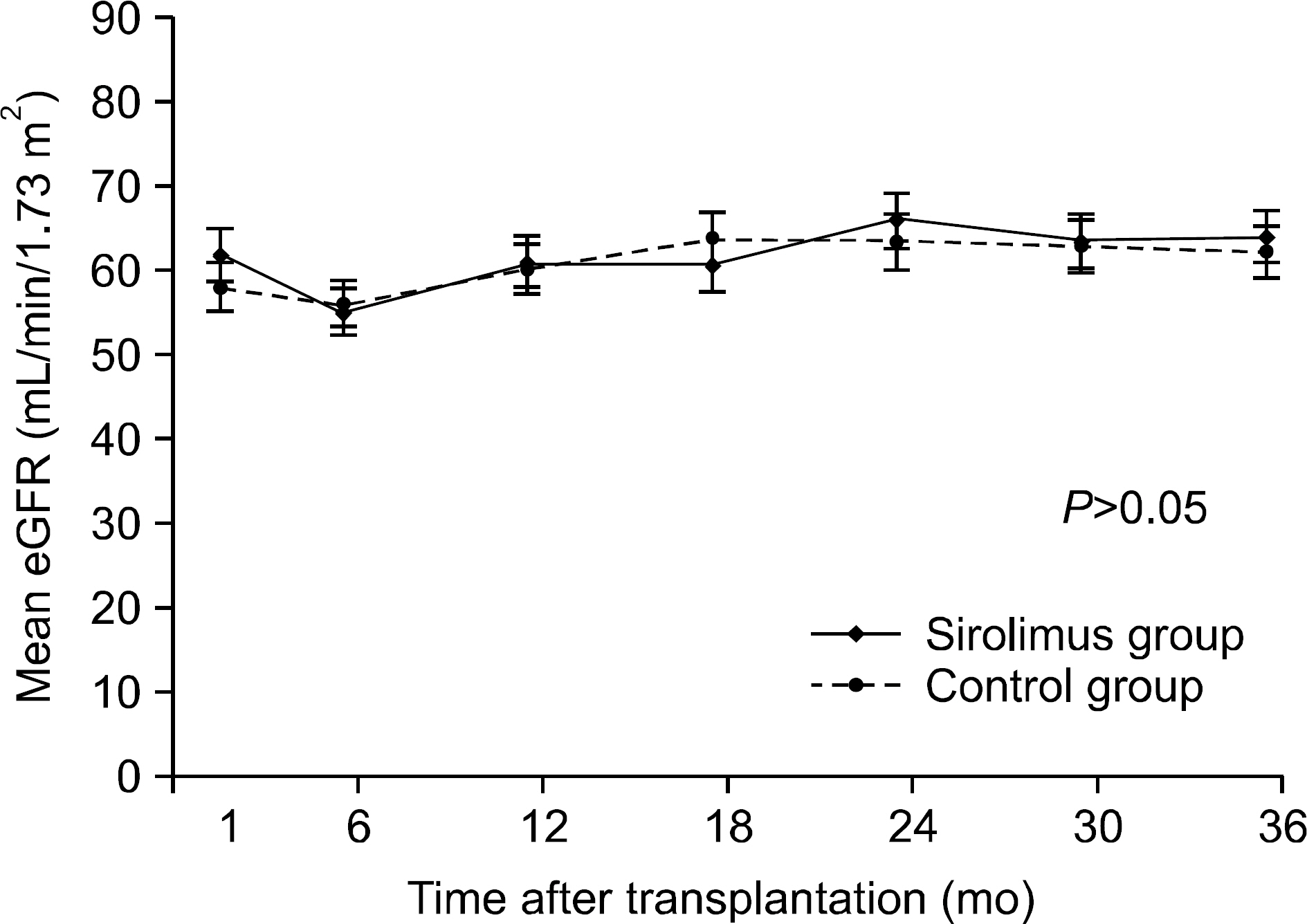
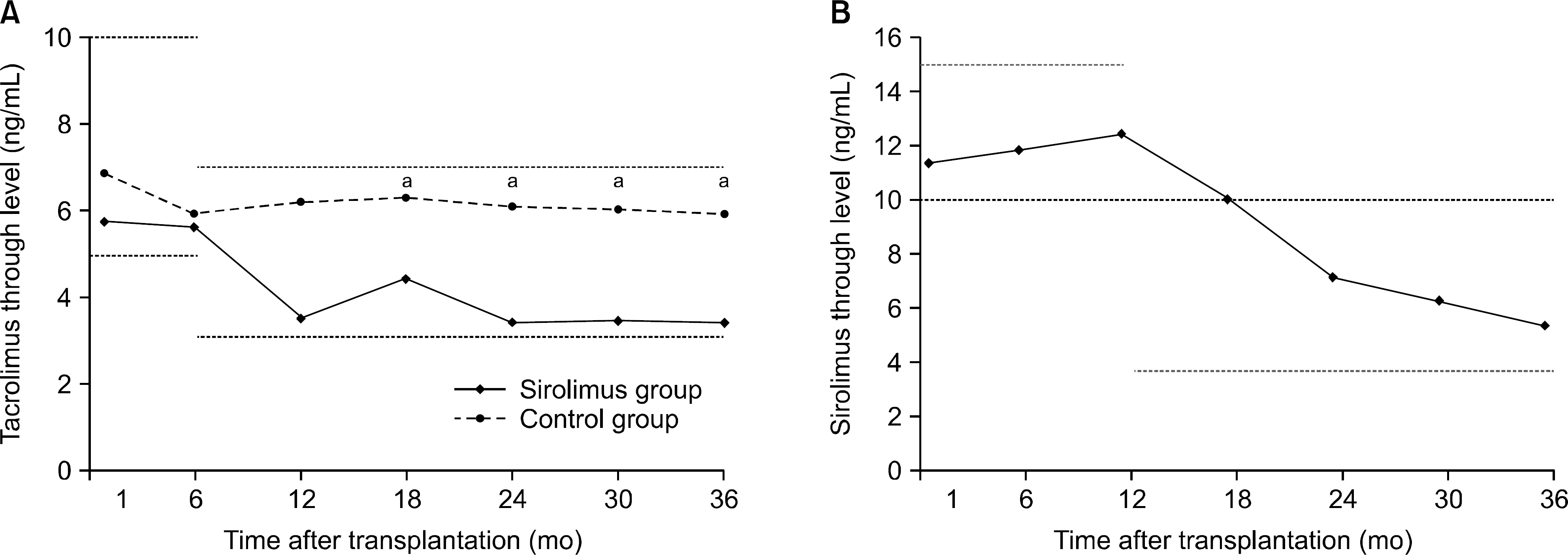
Overall, 43% of the sirolimus group versus 78% of the control group were still on the initial immunosuppressive regimen at 3 years (P=0.005), and most discontinuations in each group were due to adverse events. No differences were observed between two groups with respect to acute rejection. The mean glomerular filtration rate at 36 months was greater in the sirolimus group than in the control group, but this was not statistically significant (64.0±6.8 mL/min/1.73 m² vs. 61.8±17.1 mL/min/1.73 m², P=0.576). Graft and patient survival were similar in both groups. Importantly, mean tacrolimus through levels were significantly lower in the sirolimus group than in the control group at each time point. No neoplasm was reported in the sirolimus group. In the control group, three cases of neoplasms developed during the study period.
CONCLUSIONS
The sirolimus group had a greater number of discontinuations, particularly related to adverse events. Nevertheless, optimal concentration of sirolimus allowed reduced calcineurin inhibitor exposure in high immunologic risk patients, without increasing the risk of acute rejection and graft failure.
MeSH Terms
Figure
Reference
-
1). Lamb KE., Lodhi S., Meier-Kriesche HU. Longterm renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011. 11:450–62.
Article2). Lodhi SA., Lamb KE., Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the longterm does not mirror the dramatic short-term success. Am J Transplant. 2011. 11:1226–35.
Article3). Ekberg H., Tedesco-Silva H., Demirbas A., Vitko S., Nashan B., Gurkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007. 357:2562–75.
Article4). Gaber AO., Kahan BD., Van Buren C., Schulman SL., Scarola J., Neylan JF, et al. Comparison of sirolimus plus tacrolimus versus sirolimus plus cyclosporine in high-risk renal allograft recipients: results from an open-label, randomized trial. Transplantation. 2008. 86:1187–95.
Article5). Zaza G., Granata S., Tomei P., Masola V., Gambaro G., Lupo A. mTOR inhibitors and renal allograft: Yin and Yang. J Nephrol. 2014. 27:495–506.
Article6). Richards KR., Hager D., Muth B., Astor BC., Kaufman D., Djamali A. Tacrolimus trough level at discharge predicts acute rejection in moderately sensitized renal transplant recipients. Transplantation. 2014. 97:986–91.
Article7). Lee J., Lee JJ., Kim BS., Lee JG., Huh KH., Park Y, et al. A 12-month single arm pilot study to evaluate the efficacy and safety of sirolimus in combination with tacrolimus in kidney transplant recipients at high immunologic risk. J Korean Med Sci. 2015. 30:682–7.
Article8). Meier-Kriesche HU., Chu AH., David KM., Chi-Burris K., Steffen BJ. Switching immunosuppression medications after renal transplantation: a common practice. Nephrol Dial Transplant. 2006. 21:2256–62.9). Matas AJ., Smith JM., Skeans MA., Thompson B., Gustafson SK., Stewart DE, et al. OPTN/SRTR 2013 annual data report: kidney. Am J Transplant. 2015. 15(Suppl 2):1–34.
Article10). Lim WH., Eris J., Kanellis J., Pussell B., Wiid Z., Witcombe D, et al. A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant. 2014. 14:2106–19.
Article11). Halleck F., Duerr M., Waiser J., Huber L., Matz M., Brakemeier S, et al. An evaluation of sirolimus in renal transplantation. Expert Opin Drug Metab Toxicol. 2012. 8:1337–56.
Article12). Anil Kumar MS., Irfan Saeed M., Ranganna K., Malat G., Sustento-Reodica N., Kumar AM, et al. Comparison of four different immunosuppression protocols without longterm steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transpl Immunol. 2008. 20:32–42.
Article13). Chhabra D., Skaro AI., Leventhal JR., Dalal P., Shah G., Wang E, et al. Longterm kidney allograft function and survival in prednisone-free regimens: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Clin J Am Soc Nephrol. 2012. 7:504–12.
Article14). Guerra G., Ciancio G., Gaynor JJ., Zarak A., Brown R., Hanson L, et al. Randomized trial of immunosuppressive regimens in renal transplantation. J Am Soc Nephrol. 2011. 22:1758–68.
Article15). Tedesco Silva H Jr., Cibrik D., Johnston T., Lackova E., Mange K., Panis C, et al. Everolimus plus reduced-exposure CsA versus mycophenolic acid plus standard-exposure CsA in renal-transplant recipients. Am J Transplant. 2010. 10:1401–13.16). Kahan BD., Julian BA., Pescovitz MD., Vanrenterghem Y., Neylan J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation. 1999. 68:1526–32.17). Shihab F., Christians U., Smith L., Wellen JR., Kaplan B. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014. 31:22–32.
Article18). Engels EA., Pfeiffer RM., Fraumeni JF Jr., Kasiske BL., Israni AK., Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011. 306:1891–901.
Article19). Euvrard S., Morelon E., Rostaing L., Goffin E., Brocard A., Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012. 367:329–39.
Article20). Luan FL., Ding R., Sharma VK., Chon WJ., Lagman M., Suthanthiran M. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003. 63:917–26.21). Kim MH., Kim MS., Cha YJ., Park KS., Kim BS., Lee S. Successful sirolimus treatment of multiple visceral Kaposi's sarcoma in a renal allograft patient. J Korean Soc Transplant. 2012. 26:293–8.
Article22). Fantus D., Rogers NM., Grahammer F., Huber TB., Thomson AW. Roles of mTOR complexes in the kidney: implications for renal disease and transplantation. Nat Rev Nephrol. 2016. 12:587–609.
Article23). Kaplan B., Qazi Y., Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando). 2014. 28:126–33.
Article24). Lee HS., Huh KH., Kim YS., Kim MS., Kim HJ., Kim SI, et al. Sirolimus-induced pneumonitis after renal transplantation: a single-center experience. Transplant Proc. 2012. 44:161–3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A 12-Month Single Arm Pilot Study to Evaluate the Efficacy and Safety of Sirolimus in Combination with Tacrolimus in Kidney Transplant Recipients at High Immunologic Risk
- Impact of tacrolimus intra-patient variability on kidney transplant outcomes according to immunologic risk
- Long-term clinical outcomes of late conversion to once-daily tacrolimus and sirolimus combination in stable kidney transplant recipients
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- A Pilot Study of Calcineurin Inhibitors (CNIs) and Steroid Avoidance Immunosuppressive Protocol among Living Donor Kidney Transplant Recipients