J Vet Sci.
2016 Mar;17(1):79-87. 10.4142/jvs.2016.17.1.79.
Effect of autologous platelet-rich plasma application on cutaneous wound healing in dogs
- Affiliations
-
- 1Institute of Animal Medicine, College of Veterinary Medicine, Gyeongsang National University, Jinju 52828, Korea. jungdi@gnu.ac.kr
- 2Laboratory of Veterinary Dermatology and Neurology, College of Veterinary Medicine, Chungbuk National University, Cheongju 28644, Korea.
- 3Family Medicine Clinic and Research Institute of Convergence of Biomedical Sciences and Technology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan 50612, Korea. dwjeong75@hanmail.net
- KMID: 2363338
- DOI: http://doi.org/10.4142/jvs.2016.17.1.79
Abstract
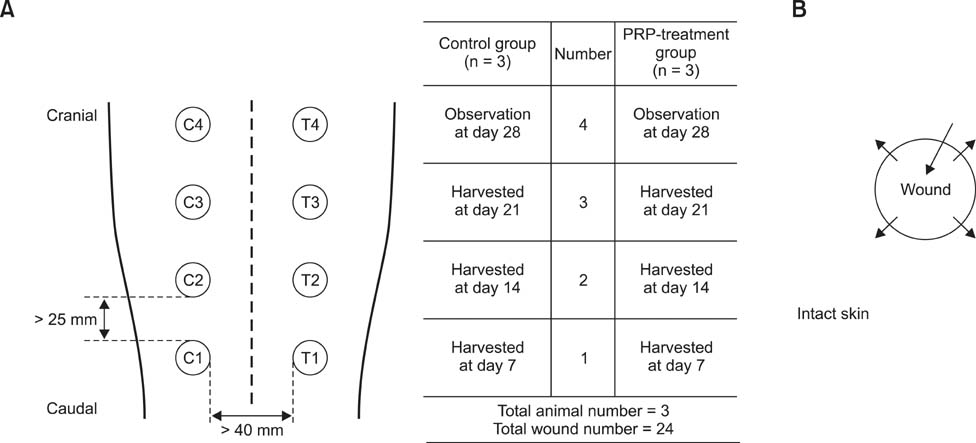
- This study was conducted to identify the effectiveness of platelet-rich plasma (PRP) and efficacy of intralesional injection as a method of application to acute cutaneous wounds in dogs. Healthy adult beagles (n = 3) were used in this study. Autologous PRP was separated from anticoagulant treated whole blood in three dogs. Cutaneous wounds were created and then treated by intralesional injection of PRP in the experimental group, while they were treated with saline in the control group on days 0, 2 and 4. The healing process was evaluated by gross examination throughout the experimental period and histologic examination on day 7, 14 and 21. In PRP treated wounds, the mean diameter was smaller and the wound closure rate was higher than in the control. Histological study revealed that PRP treated wounds showed more granulation formation and angiogenesis on day 7, and faster epithelialization, more granulation formation and collagen deposition were observed on day 14 than in control wounds. On day 21, collagen deposition and epithelialization were enhanced in PRP treated groups. Overall, PRP application showed beneficial effects in wound healing, and intralesional injection was useful for application of PRP and could be a good therapeutic option for wound management in dogs.
MeSH Terms
-
Animals
Collagen/metabolism
Dermis/cytology/injuries/physiology
Dogs
Epidermis/cytology/injuries/*physiology
Female
Granulation Tissue/cytology
Injections, Intralesional/veterinary
Male
Neovascularization, Physiologic
*Platelet-Rich Plasma
Regeneration
Treatment Outcome
*Wound Healing
Wounds and Injuries/therapy/*veterinary
Collagen
Figure
Reference
-
1. AL-Bayati AH, Al-Asadi RN, Mahdi AK, Al-Falahi NH. Effects of autologous platelets rich plasma on full-thickness cutaneous wounds healing in goats. Int J Anim Vet Adv. 2013; 5:233–239.
Article2. Alishahi MK, Mofidpoor H, Alishahi MAK. Histopathological evaluation of the effect of platelet-rich fibrin on canine cutaneous incisional wound healing. World Appl Sci J. 2014; 31:676–680.3. Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009; 91:987–996.4. Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993; 165:728–737.
Article5. Brissett AE, Hom DB. The effects of tissue sealants, platelet gels, and growth factors on wound healing. Curr Opin Otolaryngol Head Neck Surg. 2003; 11:245–250.
Article6. Carter K. Growth factors: the wound healing therapy of the future. Br J Community Nurs. 2003; 8:S15–S16. S18–S19. S22–S23.
Article7. DeRossi R, Coelho ACAO, de Mello GS, Frazílio FO, Leal CRB, Facco GG, Brum KB. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras. 2009; 24:276–281.
Article8. Dionyssiou D, Demiri E, Foroglou P, Cheva A, Saratzis N, Aivazidis C, Karkavelas G. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: an experimental and clinical study. Int Wound J. 2013; 10:397–406.
Article9. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 114:1502–1508.
Article10. Fathi WK. The effect of hyaluronic acid and platelet-rich plasma on soft tissue wound healing: an experimental study on rabbits. Al–Rafidain Dent J. 2012; 12:115–125.
Article11. Ferdousy RN, Rahman MM, Paul S, Khan MAHNA. Role of platelet rich plasma gel in the wound healing of black Bengal goat. IOSR J Agri Vet Sci. 2013; 6:14–21.
Article12. Ferguson M, Byrnes C, Sun L, Marti G, Bonde P, Duncan M, Harmon JW. Wound healing enhancement: electroporation to address a classic problem of military medicine. World J Surg. 2005; 29:Suppl 1. S55–S59.
Article13. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009; 37:2259–2272.14. Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008; 66:684–690.
Article15. Hedlund CS. Surgery of the Integumentary System. In : Fossum TW, Hedlund CS, Johnson AL, Schulz KS, Seim HB, Willard MD, Bahr A, Carroll GL, editors. Small Animal Surgery. 3rd ed. St. Louis: Mosby Elsevier;2007. p. 159–170.16. Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007; 9:174–183.
Article17. Johnston DE. Wound healing in skin. Vet Clin North Am Small Anim Pract. 1990; 20:1–25.
Article18. Kim JH, Park C, Park HM. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet Dermatol. 2009; 20:123–126.
Article19. Kimura A, Ogata H, Yazawa M, Watanabe N, Mori T, Nakajima T. The effects of platelet-rich plasma on cutaneous incisional wound healing in rats. J Dermatol Sci. 2005; 40:205–208.
Article20. Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997; 276:75–81.
Article21. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004; 62:489–496.
Article22. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85:638–646.23. Monteiro SO, Lepage OM, Theoret CL. Effects of platelet-rich plasma on the repair of wounds on the distal aspect of the forelimb in horses. Am J Vet Res. 2009; 70:277–282.
Article24. Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991; 45:319–326.
Article25. Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Pannonica Adriat. 2007; 16:156–165.26. Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. 2010; 89:961–969.
Article27. Sardari K, Emami MR, Kazemi H, Movasagi AR, Goli AA, Lotfi A, Malekzadeh S. Effects of platelet-rich plasma (PRP) on cutaneous regeneration and wound healing in dogs treated with dexamethasone. Comp Clin Pathol. 2011; 20:155–162.
Article28. Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999; 341:738–746.
Article29. Smith RG, Gassmann CJ, Campbell MS. Platelet-rich plasma: properties and clinical applications. J Lancaster Gen Hosp. 2007; 2:73–78.30. Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, Kadosawa T, Fujinaga T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999; 20:1407–1414.
Article31. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003; 83:835–870.
Article32. Willemsen JC, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014; 38:1057–1063.
Article33. Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, Kim CS, Kim BS. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med. 2011; 43:622–629.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Recalcitrant Cutaneous Ulcer of Comorbid Patient Treated with Platelet Rich Plasma: A Case Report
- Acceleration of Wound Healing Using Adipose-derived Stem Cell Therapy with Platelet Concentrates: Platelet-rich Plasma (PRP) vs. Platelet-rich Fibrin (PRF)
- Intralesional Injection of Autologous Platelet-Rich Plasma as an Effective Regeneration Therapy: A Case Report of Chronic Wagner Grade 2 Diabetic Foot Ulcer
- The Effect of Platelet Rich Plasma Combined with Bovine Bone on the Treatment of Grade II Furcation Defects in Beagle Dogs