J Vet Sci.
2016 Mar;17(1):35-44. 10.4142/jvs.2016.17.1.35.
The association of Hsp90 expression induced by aspirin with anti-stress damage in chicken myocardial cells
- Affiliations
-
- 1College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China. b_endong@njau.edu.cn
- 2Institute for Animal Hygiene, Animal Welfare and Farm Animal Behaviour, University of Veterinary Medicine Hannover, Hannover 30173, Germany.
- KMID: 2363333
- DOI: http://doi.org/10.4142/jvs.2016.17.1.35
Abstract
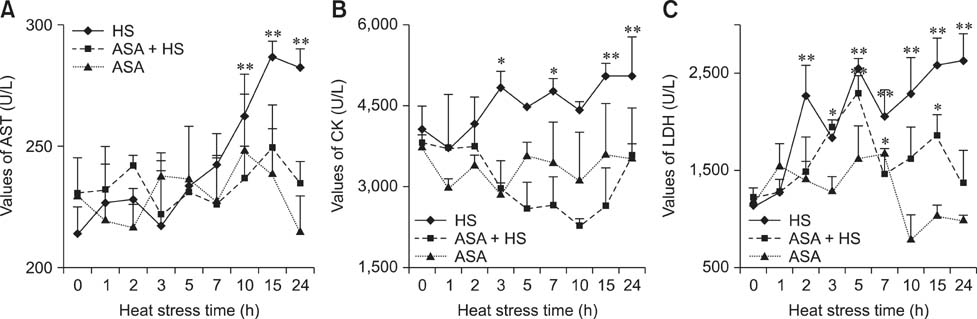
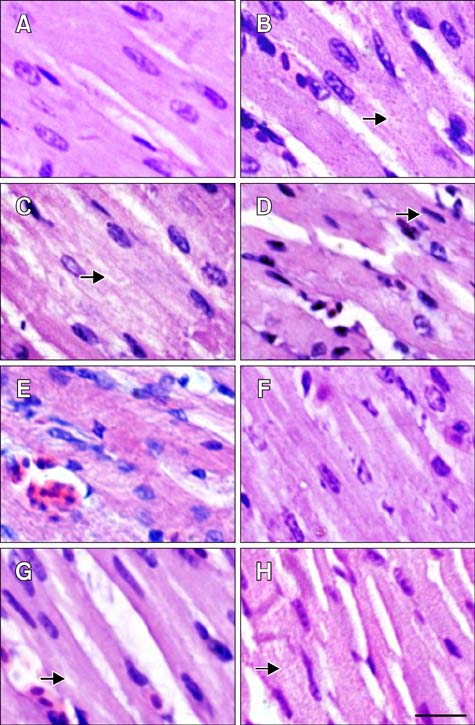
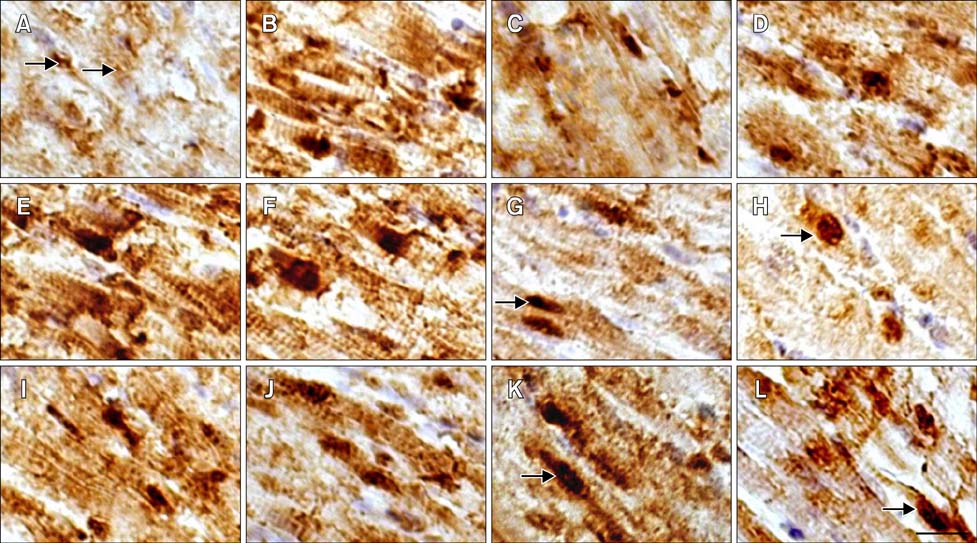
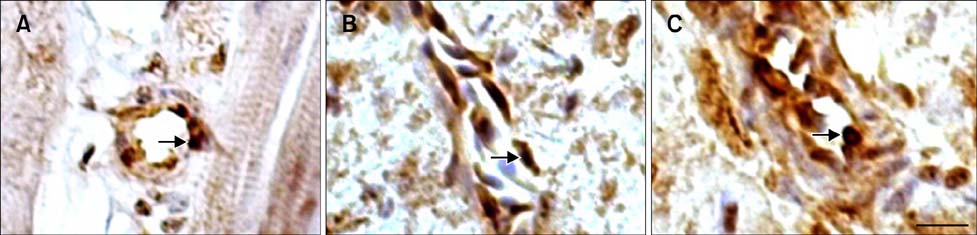
- The protective effect of aspirin during exposure to heat stress in broiler chickens was investigated. We assayed pathological damage, expression and distribution of Hsp90 protein and hsp90 mRNA expression in chicken heart tissues after oral administration of aspirin following exposure to high temperature for varying times. Heat stress induced increases in plasma aspartate aminotransferase, creatine kinase and lactate dehydrogenase activities while causing severe heart damage, which was characterized by granular and vacuolar degeneration, nuclear shrinkage and even myocardium fragmentation in cardiac muscle fibers. After aspirin administration, myocardial cells showed fewer pathological lesions than broilers treated with heat alone. A high positive Hsp90 signal was always detected in the nuclei of myocardial cells from broilers treated with aspirin, while in myocardial cells treated with heat alone, Hsp90 in the nuclei decreased, as did that in the cytoplasm. Aspirin induced rapid and significant synthesis of Hsp90 before and at the initial phase of heat stress, and significant expression of hsp90 mRNA was stimulated throughout the experiment when compared with cells exposed to heat stress alone. Thus, specific pre-induction of Hsp90 in cardiovascular tissue was useful for resisting heat stress damage because it produced stable damage-related enzymes and fewer pathologic changes.
Keyword
MeSH Terms
-
Animals
Anti-Inflammatory Agents, Non-Steroidal/pharmacology
Aspirin/*pharmacology
Cell Nucleus/genetics
Chickens
Gene Expression Regulation/*drug effects
HSP90 Heat-Shock Proteins/*genetics
Hot Temperature
Myocytes, Cardiac/*drug effects/enzymology/pathology
Stress, Physiological/*drug effects
Aspirin
Anti-Inflammatory Agents, Non-Steroidal
HSP90 Heat-Shock Proteins
Figure
Reference
-
1. Alderman BM, Cook GA, Familari M, Yeomans ND, Giraud AS. Resistance to apoptosis is a mechanism of adaptation of rat stomach to aspirin. Am J Physiol Gastrointest Liver Physiol. 2000; 278:G839–G846.
Article2. Amici C, Rossi A, Santoro MG. Aspirin enhances thermotolerance in human erythroleukemic cells: an effect associated with the modulation of the heat response. Cancer Res. 1995; 55:4452–4457.3. Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001; 17:509–519.
Article4. Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008; 13:459–466.
Article5. Bao ED, Sultan KR, Nowak B, Hartung J. Localization and expression of heat shock proteins in liver of transport stressed pigs. Zhongguo nong ye ke xue. 2002; 35:1130–1133.6. Buriro R, Lv YJ, Ali I, Tang S, Liu ZJ, Zhang M, Adem A, Hartung J, Bao ED. Temporal variations of Hsp60 and HSF-1 in primary rat myocardial cells in vitro under heat stress. Genet Mol Res. 2013; 12:3003–3016.
Article7. Csermely P, Schnaider T, Soti C, Prohászka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998; 79:129–168.8. Geraert PA, Guillaumin S, Leclercq B. Are genetically lean broilers more resistant to hot climate? Br Poult Sci. 1993; 34:643–653.
Article9. Geraert PA, Padilha JCF, Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 1996; 75:195–204.
Article10. Giardina C, Lis JT. Sodium salicylate and yeast heat shock gene transcription. J Biol Chem. 1995; 270:10369–10372.
Article11. Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995; 27:1669–1678.
Article12. Islam A, Lv YJ, Abdelnasir A, Rehana B, Liu ZJ, Zhang M, Tang S, Cheng YF, Chen HB, Hartung J, Bao ED. The role of Hsp90XMLLink_XYZ in heat-induced apoptosis and cell damage in primary myocardial cell cultures of neonatal rats. Genet Mol Res. 2013; 12:6080–6091.
Article13. Jackson SE. Hsp90: structure and function. Top Curr Chem. 2013; 328:155–240.
Article14. Jin M, Otaka M, Okuyama A, Itoh S, Otani S, Odashima M, Iwabuchi A, Konishi N, Wada I, Pacheco I, Itoh H, Tashima Y, Masamune O, Watanabe S. Association of 72-kDa heat shock protein expression with adaptation to aspirin in rat gastric mucosa. Dig Dis Sci. 1999; 44:1401–1407.15. Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992; 255:1243–1245.
Article16. Kabakov AE, Gabai VL. Heat-shock proteins maintain the viability of ATP-deprived cells: what is the mechanism? Trends Cell Biol. 1994; 4:193–196.
Article17. Kamarck T, Jennings JR. Biobehavioral factors in sudden cardiac death. Psychol Bull. 1991; 109:42–75.
Article18. Klemcke HG. Responses of the porcine pituitary-adrenal axis to chronic intermittent stressor. Domest Anim Endocrinol. 1994; 11:133–149.
Article19. Koo HN, Oh SY, Kang KI, Moon DY, Kim HD, Kang HS. Modulation of HSP70 and HSP90 expression by sodium salicylate and aspirin in fish cell line CHSE-214. Zoolog Sci. 2000; 17:1275–1282.
Article20. Lee BS, Chen J, Angelidis C, Jurivich DA, Morimoto RI. Pharmacological modulation of heat shock factor 1 by anti-inflammatory drugs results in protection against stress-induced cellular damage. Proc Natl Acad Sci U S A. 1995; 92:7207–7211.
Article21. Lei L, Yu J, Bao E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br Poult Sci. 2009; 50:504–511.
Article22. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988; 22:631–677.
Article23. Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998; 18:5091–5098.
Article24. McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM. Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg. 2006; 132:72–79.
Article25. Nadeau K, Das A, Walsh CT. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem. 1993; 268:1479–1487.
Article26. Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997; 17:469–481.
Article27. Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A. 1997; 94:12949–12956.28. Ostlund G, Sonnhammer EL. Quality criteria for finding genes with high mRNA-protein expression correlation and coexpression correlation. Gene. 2012; 497:228–236.
Article29. Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990; 348:166–168.
Article30. Prohászka Z, Füst G. Immunological aspects of heat-shock proteins-the optimum stress of life. Mol Immunol. 2004; 41:29–44.
Article31. Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998; 396:336–342.
Article32. Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Lee HK, Lee BH, Ryu DY, Park JH. Arsenite-induced apoptosis is prevented by antioxidants in zebrafish liver cell line. Toxicol In Vitro. 2007; 21:870–877.
Article33. Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001; 81:1461–1497.
Article34. Tang S, Buriro R, Liu Z, Zhang M, Ali I, Adam A, Hartung J, Bao E. Localization and expression of Hsp27 and XMLLink_XYZB-crystallin in rat primary myocardial cells during heat stress in vitro. PLoS One. 2013; 8:e69066.35. van der Hel der, Versteqen MW, Pijls L, van Kampen M. Effect of two-day temperature exposure of neonatal broiler chicks on growth performance and body composition during two weeks at normal conditions. Poult Sci. 1992; 71:2014–2021.
Article36. Wada H, Kamiike W. Aspartate aminotransferase isozymes and their clinical significance. Prog Clin Biol Res. 1990; 344:853–875.37. Yu H, Bao ED, Zhao RQ, Lv QX. Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am J Vet Res. 2007; 68:1145–1150.
Article38. Yu J, Bao E, Yan J, Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones. 2008; 13:327–335.
Article39. Zhang M, Xin L, Bao E, Hartung J, Yue Z. Variation in the expression of Hsp27, XMLLink_XYZB-crystallin mRNA and protein in heart and liver of pigs exposed to different transport times. Res Vet Sci. 2011; 90:432–438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis in response to heat stress is positively associated with heat-shock protein 90 expression in chicken myocardial cells in vitro
- Protective Effect of HSP90 on Neuronal Cell Death-induced by beta-Amyloid Peptide
- Traditional and Novel Mechanisms of Heat Shock Protein 90 (HSP90) Inhibition in Cancer Chemotherapy Including HSP90 Cleavage
- Effect of Cryopreservation on the Heat Shock Protein 90 Expression in Mouse Ovarian Tissue
- Increased Expression of Prostaglandin H synthase by Aspirin in Cultured Cells from Amnionic Cell Line WISH Cells