Lab Anim Res.
2016 Dec;32(4):208-216. 10.5625/lar.2016.32.4.208.
Multilocus sequence typing analysis of Pseudomonas aeruginosa isolated from pet Chinese stripe-necked turtles (Ocadia sinensis)
- Affiliations
-
- 1Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea. gjheo@cbu.ac.kr
- KMID: 2362912
- DOI: http://doi.org/10.5625/lar.2016.32.4.208
Abstract
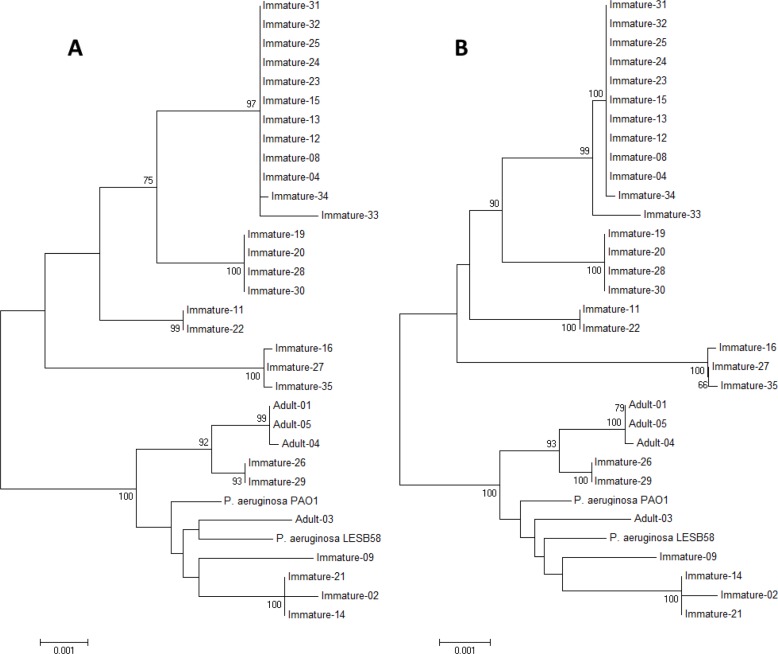
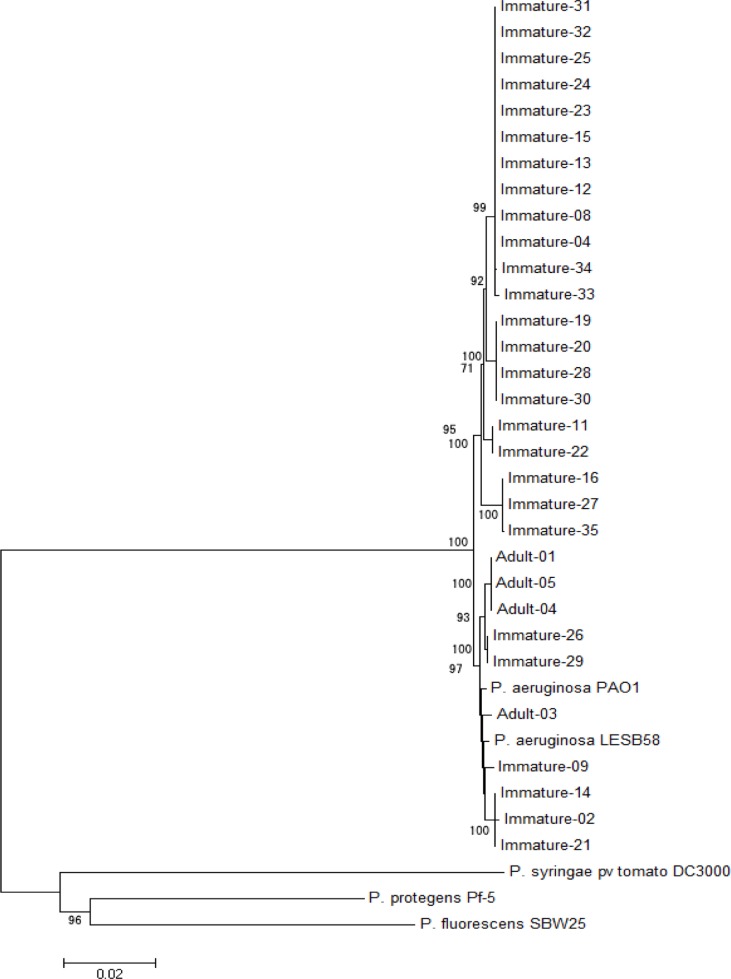
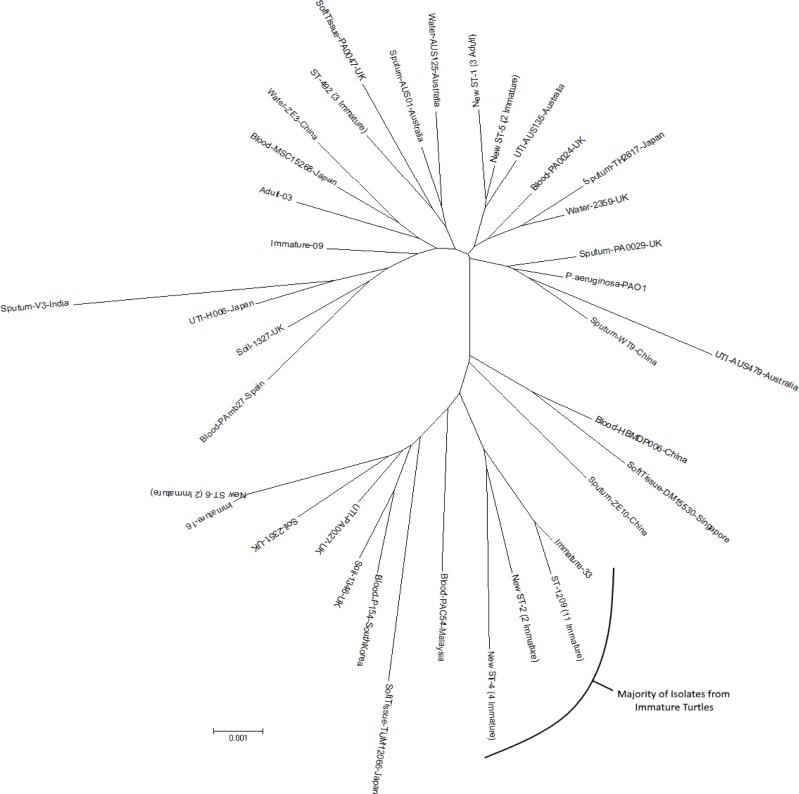
- Our research sought to characterize the phylogeny of Pseudomonas aeruginosa isolated from pet Chinese stripe-necked turtles (Ocadia sinensis) to better understand its evolutionary relation to other isolates and increase understanding of a potential zoonotic pathogen transmitted through direct contact with pet turtles. Thirty-one Pseudomonas aeruginosa isolates were obtained from both immature and adult turtles sold in pet shops in Korea. To characterize the phylogenic position of Chinese stripe-necked turtle-borne P. aeruginosa relative to other strains, multilocus sequence typing (MLST) analysis was performed due to the accessibility and breadth of MLST databases. Seven housekeeping genes (acsA, aroE, guaA, mutL, nuoD, ppsA, and trpE) were sequenced and the results were compared with data from the MLST database. The genes were further used for phylogenetic analysis of P. aeruginosa using concatenated gene fragments. Both rooted and unrooted phylogenetic trees were generated. Eleven distinct sequence types were present within the isolates among which seven were new. Expanding an unrooted phylogenetic tree to include P. aeruginosa MLST sequences isolated from various other geographic locations and sources revealed a divergent cluster containing the majority of isolates obtained from turtles. This suggests that P. aeruginosa strains particularly well-adapted for inhabiting turtles occupy a distinct phylogenetic position.
Keyword
MeSH Terms
Figure
Reference
-
1. Spiers AJ, Buckling A, Rainey PB. The causes of Pseudomonas diversity. Microbiology. 2000; 146(Pt 10):2345–2350. PMID: 11021911.
Article2. Vonberg RP, Gastmeier P. Isolation of infectious cystic fibrosis patients: results of a systematic review. Infect Control Hosp Epidemiol. 2005; 26(4):401–409. PMID: 15865277.
Article3. Bert F, Maubec E, Bruneau B, Berry P, Lambert-Zechovsky N. Multi-resistant Pseudomonas aeruginosa outbreak associated with contaminated tap water in a neurosurgery intensive care unit. J Hosp Infect. 1998; 39(1):53–62. PMID: 9617685.
Article4. Becks VE, Lorenzoni NM. Pseudomonas aeruginosa outbreak in a neonatal intensive care unit: a possible link to contaminated hand lotion. Am J Infect Control. 1995; 23(6):396–398. PMID: 8821117.
Article5. Buttery JP, Alabaster SJ, Heine RG, Scott SM, Crutchfield RA, Bigham A, Tabrizi SN, Garland SM. Multiresistant Pseudomonas aeruginosa outbreak in a pediatric oncology ward related to bath toys. Pediatr Infect Dis J. 1998; 17(6):509–513. PMID: 9655544.
Article6. Schmidt KD, Tummler B, Romling U. Comparative genome mapping of Pseudomonas aeruginosa PAO with P. aeruginosa C, which belongs to a major clone in cystic fibrosis patients and aquatic habitats. J Bacteriol. 1996; 178(1):85–93. PMID: 8550447.
Article7. Mathee K, Narasimhan G, Valdes C, Qiu X, Matewish JM, Koehrsen M, Rokas A, Yandava CN, Engels R, Zeng E, Olavarietta R, Doud M, Smith RS, Montgomery P, White JR, Godfrey PA, Kodira C, Birren B, Galagan JE, Lory S. Dynamics of Pseudomonas aeruginosa genome evolution. Proc Natl Acad Sci U S A. 2008; 105(8):3100–3105. PMID: 18287045.
Article8. Pirnay JP, Bilocq F, Pot B, Cornelis P, Zizi M, Van Eldere J, Deschaght P, Vaneechoutte M, Jennes S, Pitt T, De Vos D. Pseudomonas aeruginosa population structure revisited. PLoS One. 2009; 4(11):e7740. PMID: 19936230.
Article9. Alonso A, Rojo F, Martinez JL. Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ Microbiol. 1999; 1(5):421–430. PMID: 11207762.
Article10. Pirnay JP, Matthijs S, Colak H, Chablain P, Bilocq F, Van Eldere J, De Vos D, Zizi M, Triest L, Cornelis P. Global Pseudomonas aeruginosa biodiversity as reflected in a Belgian river. Environ Microbiol. 2005; 7(7):969–980. PMID: 15946293.
Article11. Enright MC, Spratt BG. Multilocus sequence typing. Trends Microbiol. 1999; 7(12):482–487. PMID: 10603483.
Article12. Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, Zhang Q, Zhou J, Zurth K, Caugant DA, Feavers IM, Achtman M, Spratt BG. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998; 95(6):3140–3145. PMID: 9501229.
Article13. Waters V, Zlosnik JE, Yau YC, Speert DP, Aaron SD, Guttman DS. Comparison of three typing methods for Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2012; 31(12):3341–3350. PMID: 22843295.
Article14. Bonin F, Devaux B, Dupré A. Turtles of the World. London: A and C Black;2006.15. Warwick C, Arena PC, Steedman C, Jessop M. A review of captive exotic animal-linked zoonoses. J Environ Health Res. 2012; 12(1):9–24.16. Centers for Disease Control and Prevention (CDC). Salmonellosis associated with pet turtles--Wisconsin and Wyoming, 2004. MMWR Morb Mortal Wkly Rep. 2005; 54(9):223–226. PMID: 15758895.17. Centers for Disease Control and Prevention (CDC). Turtle-associated salmonellosis in humans--United States, 2006-2007. MMWR Morb Mortal Wkly Rep. 2007; 56(26):649–652. PMID: 17615521.18. Warwick C, Arena PC, Steedman C. Health implications associated with exposure to farmed and wild sea turtles. JRSM Short Rep. 2013; 4(1):8. PMID: 23413410.
Article19. Dickinson VM, Duck T, Schwalbe CR, Jarchow JL, Trueblood MH. Nasal and cloacal bacteria in free-ranging desert tortoises from the western United States. J Wildl Dis. 2001; 37(2):252–257. PMID: 11310875.
Article20. Divers SJ, Mader DR. Reptile Medicine and Surgery. Elsevier Health Sciences;2005. p. 6446.21. Friedrich M, Lessnau K-D, Cunha BA. Pseudomonas aeruginosa Infections: Practice Essentials, Background, Pathophysiology. 2015. 12. 27. cited 2016 Apr 19. Available from: http://emedicine.medscape.com/article/226748-overview.22. Bluvias JE, Eckert KL. Marine Turtle Trauma Response Procedures: A Husbandry Manual. Wider Caribbean Sea Turtle Conservation Network (WIDECAST);2010.23. Curran B, Jonas D, Grundmann H, Pitt T, Dowson CG. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J Clin Microbiol. 2004; 42(12):5644–5649. PMID: 15583294.24. Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016; 33(7):1870–1874. PMID: 27004904.
Article25. Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980; 16(2):111–120. PMID: 7463489.
Article26. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4(4):406–425. PMID: 3447015.27. U.S. Food and Drug Administration (FDA). FDA Acts to Reduce Risk of Salmonella Infections. 2008.28. Gomila M, Del Carmen Gallegos M, Fernandez-Baca V, Pareja A, Pascual M, Diaz-Antolin P, Garcia-Valdes E, Lalucat J. Genetic diversity of clinical Pseudomonas aeruginosa isolates in a public hospital in Spain. BMC Microbiol. 2013; 13:138. PMID: 23773707.
Article29. Chen SH, Chen RY, Xu XL, Chen HT. Multilocus sequencing typing of Pseudomonas aeruginosa isolates and analysis of potential pathogenicity of typical genotype strains from occupational oxyhelium saturation divers. Undersea Hyperb Med. 2014; 41(2):135–141. PMID: 24851551.30. Khan NH, Ahsan M, Yoshizawa S, Hosoya S, Yokota A, Kogure K. Multilocus sequence typing and phylogenetic analyses of Pseudomonas aeruginosa Isolates from the ocean. Appl Environ Microbiol. 2008; 74(20):6194–6205. PMID: 18757570.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial property of lemongrass (Cymbopogon citratus) oil against pathogenic bacteria isolated from pet turtles
- Dissemination of Carbapenem-Resistance among Multidrug Resistant Pseudomonas aeruginosa carrying Metallo-Beta-Lactamase Genes, including the Novel bla(IMP-65) Gene in Thailand
- Mechanisms of ceftolozane/tazobactam resistance in Pseudomonas aeruginosa isolates from South Korea: A diagrostic study
- Phenotypic characterization of pseudomonas aeruginosa by pyocin typing of two different methods
- Prevalence of Salmonella spp. in pet turtles and their environment