J Korean Med Sci.
2016 Jan;31(1):1-8. 10.3346/jkms.2016.31.1.1.
Review of the Registration in the Clinical Research Information Service
- Affiliations
-
- 1Division of Cardiovascular and Rare Disease, Center for Biomedical Science, Korea National Institute of Health, Cheongju, Korea. hypark65@korea.kr
- KMID: 2359984
- DOI: http://doi.org/10.3346/jkms.2016.31.1.1
Abstract
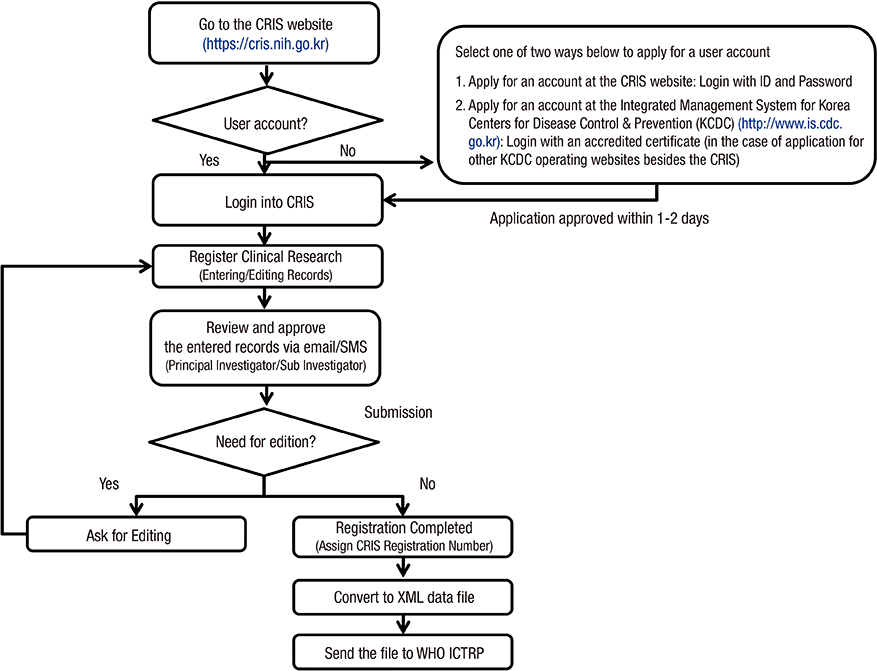
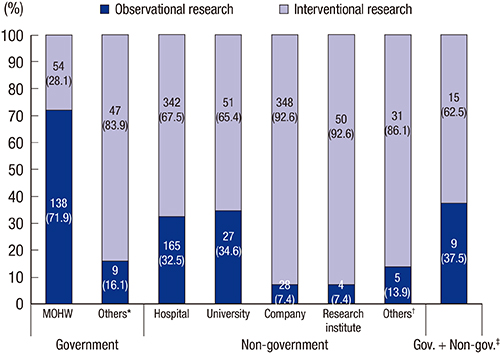
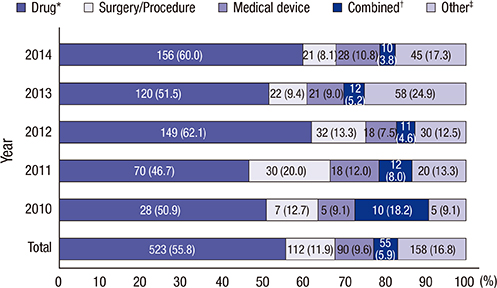
- Clinical research registration is required in many countries to improve transparency of clinical research and to ensure subject safety. Developed in February 2010, the Clinical Research Information Service (CRIS) is an online registration system for clinical studies in Korea and one of the primary registries of the World Health Organization (WHO) International Clinical Trials Registry Platform. The present analysis investigated the characteristics of studies registered in the CRIS between February 2010 and December 2014. Data for the analysis were extracted from the CRIS database. As of December 31, 2014, 1,323 clinical studies were registered. Of these, 938 (70.9%) were interventional studies and 385 (29.1%) were observational studies. A total of 248 (18.7%) studies were funded by government sources, 1,051 (79.4%) by non-government sources, and 24 (1.8%) by both. The most frequently studied disease category based on the ICD-10 classification was the digestive system (13.1%), followed by the nervous system (9.4%) and musculoskeletal system (9.1%). Only 17.8% of the studies were registered prior to enrollment of the first subject. Comparing the number of registered or approved clinical studies between the CRIS, the Ministry of Food and Drug Safety, and ClinicalTrials.gov suggests that a considerable number of clinical studies are not registered with the CRIS; therefore, we would suggest that such registration should be the mandatory legal requirement.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Underregistration and Underreporting of Stem Cell Clinical Trials in Neurological Disorders
Timothy E. Lee, Aryun Kim, Mihee Jang, Beomseok Jeon
J Clin Neurol. 2018;14(2):215-224. doi: 10.3988/jcn.2018.14.2.215.
Reference
-
1. Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol. 1986; 4:1529–1541.2. Chan AW. Bias, spin, and misreporting: time for full access to trial protocols and results. PLoS Med. 2008; 5:e230.3. Dickersin K, Min YI. Publication bias: the problem that won't go away. Ann N Y Acad Sci. 1993; 703:135–146.4. Dickersin K, Rennie D. Registering clinical trials. JAMA. 2003; 290:516–523.5. Viergever RF, Karam G, Reis A, Ghersi D. The quality of registration of clinical trials: still a problem. PLoS One. 2014; 9:e84727.6. Park HY. Primary registry of the WHO International Clinical Trial Registry Platform: Clinical Research Information Service. J Korean Med Assoc. 2011; 54:92–97.7. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, Kotzin S, Laine C, Marusic A, Overbeke AJ, et al. International Committee of Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004; 351:1250–1251.8. World Medical Association. Declaration of Helsinki (2013). accessed on 19 March 2015. Available at http://www.wma.net/en/30publications/10policies/b3/.9. WHO. Organizations with policies on clinical trial registration in WHO International Clinical Trials Registry Platform. accessed on 19 March 2015. Available at http://www.who.int/ictrp/trial-reg/en/index2.html.10. WHO. WHO International Clinical Trials Registry Platform primary registries. accessed on 19 March 2015. Available at http://www.who.int/ictrp/network/primary/en/.11. WHO. WHO International Clinical Trials Registry Platform. accessed on 19 March 2015. Available at http://who.int/ictrp/en/.12. Korea Ministry of Government Legislation. Regulation for management of health and medical technology R&D projects. accessed on 19 March 2015. Available at http://www.law.go.kr/admRulLsInfoP.do?admRulSeq=2100000002930.13. Ministry of Health and Welfare, Korea Health Industry Development Institute. Condition and method for application, Grant announcement of health and medical technology R&D projects (stem cell and regenerative medicine). Cheongju: Korea Health Industry Development Institute;2015. p. 25.14. Ministry of Food and Drug Safety. The official annual brief regarding the approved clinical trials in 2013. accessed on 19 March 2015. Available at http://www.mfds.go.kr/index.do?mid=664&pageNo=46&seq=22851&cmd=v.15. Ministry of Food and Drug Safety. The official annual brief regarding the approved clinical trials in 2014. accessed on 19 March 2015. Available at http://www.mfds.go.kr/index.do?mid=664&pageNo=5&seq=26363&cmd=v.16. Food and Drug Administration. Food and Drug Administration Modernization Act of 1997. accessed on 10 April 2015. Available at http://www.gpo.gov/fdsys/pkg/PLAW-105publ115/pdf/PLAW-105publ115.pdf#page=16.17. Food and Drug Administration. Food and Drug Administration Amendment Act of 2007. accessed on 19 March 2015. Available at http://gpo.gov/fdsys/pkg/PLAW-110publ85/html/PLAW-110publ85.htm.18. WHO. WHO International Clinical Trials Registry Platform organizations with policy. accessed on 11 May 2015. Available at http://www.who.int/ictrp/trial_reg/en/index2.html.19. Clinical Trials Registry-India (CTRI). Frequently Asked Questions. accessed on 11 May 2015. Available at http://ctri.nic.in/Clinicaltrials/faq.php#5a.20. Brazilian Clinical Trials Registry (ReBec). About the ReBec. accessed on 11 May 2015. Available at http://www.ensaiosclinicos.gov.br/about/.21. EU Clinical Trials Register. Legal basis. accessed on 11 May 2015. Available at https://www.clinicaltrialsregister.eu/about.html.22. Manchikanti L, Benyamin RM, Helm S 2nd, Hirsch JA. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: part 3: systematic reviews and meta-analyses of randomized trials. Pain Physician. 2009; 12:35–72.23. Hudson KL, Collins FS. Sharing and reporting the results of clinical trials. JAMA. 2015; 313:355–356.24. Alley AB, Seo JW, Hong ST. Reporting results of research involving human subjects: an ethical obligation. J Korean Med Sci. 2015; 30:673–675.25. WHO. WHO statement on public disclosure of clinical trials results. accessed on 11 May 2015. Available at http://www.who.int/ictrp/results/reporting/en/.26. National Institute of Food and Drug Safety Evaluation. Survey on the awareness about clinical trial registration, Study on improvement of clinical trial registration and operation. Cheongju: National Institute of Food and Drug Safety Evaluation;2014. p. 62–63.27. Viergever RF, Karam G, Reis A, Ghersi D. The quality of registration of clinical trials: still a problem. PLoS One. 2014; 9:e84727.28. Reveiz L, Cortés-Jofré M, Asenjo Lobos C, Nicita G, Ciapponi A, Garcìa-Dieguez M, Tellez D, Delgado M, Solà I, Ospina E. Iberoamerican Cochrane Network. Influence of trial registration on reporting quality of randomized trials: study from highest ranked journals. J Clin Epidemiol. 2010; 63:1216–1222.29. Killeen S, Sourallous P, Hunter IA, Hartley JE, Grady HL. Registration rates, adequacy of registration, and a comparison of registered and published primary outcomes in randomized controlled trials published in surgery journals. Ann Surg. 2014; 259:193–196.30. Gandhi R, Jan M, Smith HN, Mahomed NN, Bhandari M. Comparison of published orthopaedic trauma trials following registration in Clinicaltrials.gov. BMC Musculoskelet Disord. 2011; 12:278.31. Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med. 2010; 153:158–166.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Registration and Reporting Guidelines for Clinical Trials
- Primary registry of the WHO International Clinical Trial Registry Platform: Clinical Research Information Service (CRIS)
- The Study on Performance Evaluation and Improvement of the Information System for the Disabled
- Investigation of Hansen's disease patient's registration system
- A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples