Korean J Urol.
2015 Dec;56(12):837-844. 10.4111/kju.2015.56.12.837.
Noxious electrical stimulation of the pelvic floor and vagina induces transient voiding dysfunction in a rabbit survival model of pelvic floor dystonia
- Affiliations
-
- 1Department of Urology, Stanford University School of Medicine, Stanford, CA, USA. adobber@stanford.edu
- 2Division of Urology, Albany Medical College, Albany, NY, USA.
- 3Stratton VA Medical Center, Albany, NY, USA.
- 4Department of Physical Medicine and Rehabilitation, Albany Medical College, Albany, NY, USA.
- KMID: 2359877
- DOI: http://doi.org/10.4111/kju.2015.56.12.837
Abstract
- PURPOSE
Existing data supports a relationship between pelvic floor dysfunction and lower urinary tract symptoms. We developed a survival model of pelvic floor dysfunction in the rabbit and evaluated cystometric (CMG), electromyographic (EMG) and ambulatory voiding behavior.
MATERIALS AND METHODS
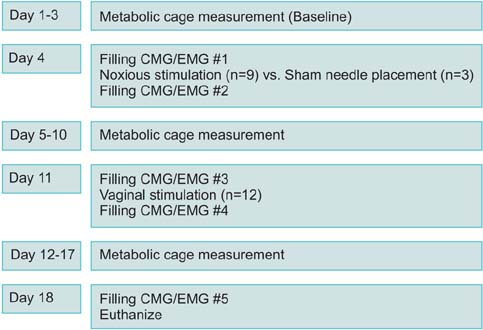
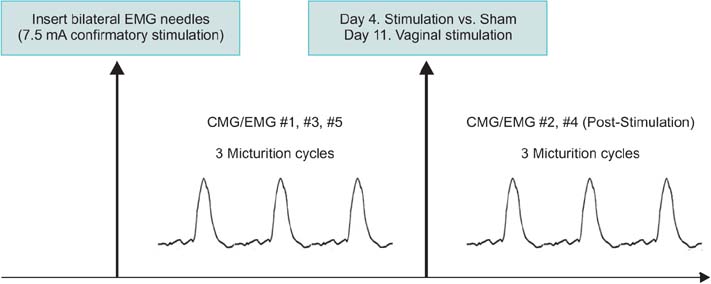
Twelve female adult virgin rabbits were housed in metabolic cages to record voiding and defecation. Anesthetized CMG/EMG was performed before and after treatment animals (n=9) received bilateral tetanizing needle stimulation to the pubococcygeous (PC) muscle and controls (n=3) sham needle placement. After 7 days all animals were subjected to tetanizing transvaginal stimulation and CMG/EMG. After 5 days a final CMG/EMG was performed.
RESULTS
Of rabbits that underwent needle stimulation 7 of 9 (78%) demonstrated dysfunctional CMG micturition contractions versus 6 of 12 (50%) after transvaginal stimulation. Needle stimulation of the PC musculature resulted in significant changes in: basal CMG pressure, precontraction pressure change, contraction pressure, interval between contractions and postvoid residual; with time to 3rd contraction increased from 38 to 53 minutes (p=0.008 vs. prestimulation). Vaginal noxious stimulation resulted in significant changes in: basal CMG pressure and interval between contractions; with time to 3rd contraction increased from 37 to 46 minutes (p=0.008 vs. prestimulation). Changes in cage parameters were primarily seen after direct needle stimulation.
CONCLUSIONS
In a majority of animals, tetanizing electrical stimulation of the rabbit pelvic floor resulted in voiding changes suggestive of pelvic floor dysfunction as characterized by a larger bladder capacity, longer interval between contractions and prolonged contraction duration.
Keyword
MeSH Terms
-
Animals
Disease Models, Animal
Dystonia/*etiology
Electric Stimulation/adverse effects/methods
Electromyography/methods
Female
Muscle Contraction/physiology
Pelvic Floor/*physiopathology
Pelvic Floor Disorders/*complications/physiopathology
Rabbits
Urinary Bladder/physiopathology
Urinary Retention/*etiology
Urination/physiology
Urine
Vagina/*physiopathology
Figure
Reference
-
1. Messelink B, Benson T, Berghmans B, Bo K, Corcos J, Fowler C, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005; 24:374–380.2. Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn. 2010; 29:77–81.3. Malykhina AP. Neural mechanisms of pelvic organ crosssensitization. Neuroscience. 2007; 149:660–672.4. Rudick CN, Chen MC, Mongiu AK, Klumpp DJ. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2007; 293:R1191–R1198.5. Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009; 144:Suppl 1. S146–S158.6. Barroso JC, Ramos JG, Martins-Costa S, Sanches PR, Muller AF. Transvaginal electrical stimulation in the treatment of urinary incontinence. BJU Int. 2004; 93:319–323.7. Alves PG, Nunes FR, Guirro EC. Comparison between two different neuromuscular electrical stimulation protocols for the treatment of female stress urinary incontinence: a randomized controlled trial. Rev Bras Fisioter. 2011; 15:393–398.8. Brubaker L, Benson JT, Bent A, Clark A, Shott S. Transvaginal electrical stimulation for female urinary incontinence. Am J Obstet Gynecol. 1997; 177:536–540.9. Dionisi B, Senatori R. Effect of transcutaneous electrical nerve stimulation on the postpartum dyspareunia treatment. J Obstet Gynaecol Res. 2011; 37:750–753.10. Martinez-Gomez M, Mendoza-Martinez G, Corona-Quintanilla DL, Fajardo V, Rodriguez-Antolin J, Castelan F. Multiparity causes uncoordinated activity of pelvic- and perinealstriated muscles and urodynamic changes in rabbits. Reprod Sci. 2011; 18:1246–1252.11. Gill BC, Moore C, Damaser MS. Postpartum stress urinary incontinence: lessons from animal models. Expert Rev Obstet Gynecol. 2010; 5:567–580.12. Fajardo V, Pacheco P, Hudson R, Jimenez I, Martinez-Gomez M. Differences in morphology and contractility of the bulbospongiosus and pubococcygeus muscles in nulliparous and multiparous rabbits. Int Urogynecol J Pelvic Floor Dysfunct. 2008; 19:843–849.13. Spettel S, Levin R, Dubin A, De E. Animal model of pelvic floor dystonia and cystometric effects on urinary dysfunction. In: Society for Urodynamics and Female Urology, 2012 Winter Meeting; 2012 Feb 28-Mar 3; New Orleans (LA), USA. Neurourol Urodyn. 2012; 31(2):234.14. Martinez-Gomez M, Lucio RA, Carro M, Pacheco P, Hudson R. Striated muscles and scent glands associated with the vaginal tract of the rabbit. Anat Rec. 1997; 247:486–495.15. Butrick CW. Pelvic floor hypertonic disorders: identification and management. Obstet Gynecol Clin North Am. 2009; 36:707–722.16. Corona-Quintanilla DL, Castelan F, Fajardo V, Manzo J, Martinez-Gomez M. Temporal coordination of pelvic and perineal striated muscle activity during micturition in female rabbits. J Urol. 2009; 181:1452–1458.17. Rajasekaran MR, Sohn D, Salehi M, Bhargava V, Fritsch H, Mittal RK. Role of puborectalis muscle in the genesis of urethral pressure. J Urol. 2012; 188:1382–1388.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pelvic floor muscle rehabilitation
- The Efficacy of Pelvic Floor Muscles Exercise Combined with Biofeedback and Electrical Stimulation in Recurred Stress In continence or Intrinsic Sphincter Dysfunction Patients
- Pelvic Floor Electrical Stimulation
- The Effect of Pelvic Floor Muscle Training with Biofeedback and Functional Electrical Stimulation for Genuine Stress Urinary Incontinence
- Biofeedback and Functional Electrical Stimulation Therapy for Patients with Intractable Chronic Pelvic Pain Syndrome