Brain Tumor Res Treat.
2016 Oct;4(2):145-149. 10.14791/btrt.2016.4.2.145.
World Health Organization Grade II Oligodendroglioma Occurring after Successful Treatment for Childhood Acute Lymphoblastic Leukemia
- Affiliations
-
- 1Department of Neurosurgery, Korea University Anam Hospital, College of Medicine, Korea University, Seoul, Korea. kyungjae99@hanmail.net
- KMID: 2356986
- DOI: http://doi.org/10.14791/btrt.2016.4.2.145
Abstract
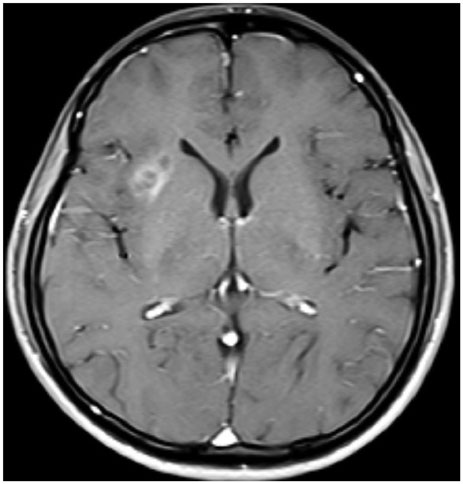
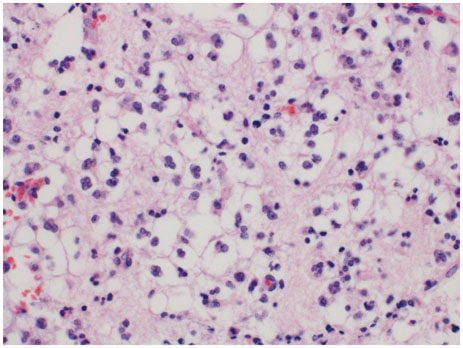
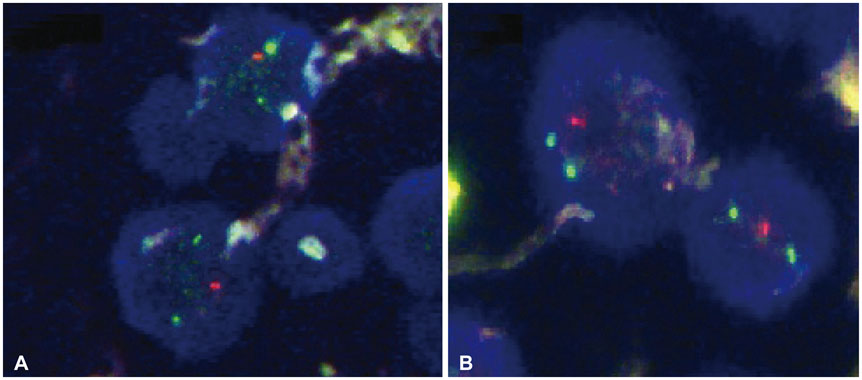
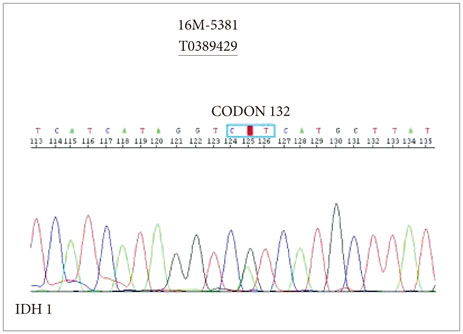
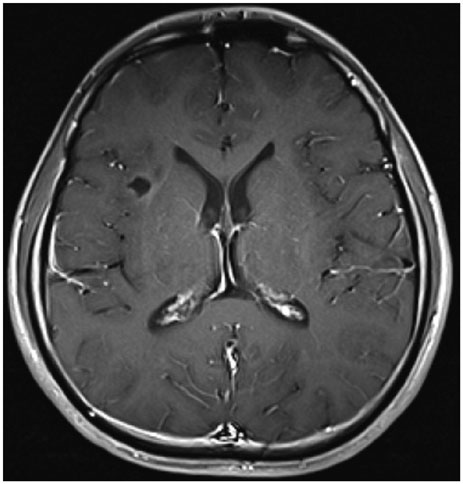
- When treating childhood acute lymphoblastic leukemia (ALL), secondary neoplasms are a significant long term problem. Radiation is generally accepted to be a major cause of the development of secondary neoplasms. Following treatment for ALL, a variety of secondary tumors, including brain tumors, hematologic malignancies, sarcomas, thyroid cancers, and skin cancers have been reported. However, oligodendroglioma as a secondary neoplasm is extremely rare. Herein we present a case of secondary oligodendroglioma occurring 13 years after the end of ALL treatment.
Keyword
MeSH Terms
Figure
Reference
-
1. Albert RE, Omran AR, Brauer EW, et al. Follow-up study of patients treated by x-ray for tinea capitis. Am J Public Health Nations Health. 1966; 56:2114–2120.
Article2. Albo V, Miller D, Leiken S, Sather H, Hammond D. Nine brain tumours (BT) as a late effect in children “cured” of acute lymphoblastic leukemia (ALL) from a single protocol study (Abstr). Proc Am Soc Clin Oncol. 1985; 4:172.3. Alexander MJ, DeSalles AA, Tomiyasu U. Multiple radiation-induced intracranial lesions after treatment for pituitary adenoma. Case report. J Neurosurg. 1998; 88:111–115.
Article4. Alexiou GA. High-grade gliomas in survivors of childhood acute lymphoblastic leukaemia. Childs Nerv Syst. 2009; 25:779. author reply 781-2.
Article5. Alexiou GA, Moschovi M, Georgoulis G, et al. Anaplastic oligodendrogliomas after treatment of acute lymphoblastic leukemia in children: report of 2 cases. J Neurosurg Pediatr. 2010; 5:179–183.
Article6. Anderson JR, Treip CS. Radiation-induced intracranial neoplasms. A report of three possible cases. Cancer. 1984; 53:426–429.
Article7. Borgmann A, Zinn C, Hartmann R, et al. Secondary malignant neoplasms after intensive treatment of relapsed acute lymphoblastic leukaemia in childhood. Eur J Cancer. 2008; 44:257–268.
Article8. D’Elia A, Melone GA, Brogna C, Formichella A, Santoro A, Salvati M. Radio-induced low-grade glioma: report of two cases and review of the literature. Neurol Sci. 2009; 30:137–141.
Article9. Dierssen G, Alvarez G, Figols J. Anaplastic astrocytomas associated with previous radiotherapy: report of three cases. Neurosurgery. 1988; 22(6 Pt 1):1095–1097.
Article10. Hah JO. Anaplastic oligodendroglioma after childhood acute lymphoblastic leukemia: chemotherapy and autologous peripheral blood stem cell transplantation. J Pediatr Hematol Oncol. 2008; 30:764–767.
Article11. Harrison MJ, Wolfe DE, Lau TS, Mitnick RJ, Sachdev VP. Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg. 1991; 75:564–574.
Article12. Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007; 99:300–308.
Article13. Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007; 297:1207–1215.
Article14. Ishida Y, Maeda M, Urayama KY, et al. Secondary cancers among children with acute lymphoblastic leukaemia treated by the Tokyo Children’s Cancer Study Group protocols: a retrospective cohort study. Br J Haematol. 2014; 164:101–112.
Article15. Jones A. Supervoltage X-ray therapy of intracranial tumours. Ann R Coll Surg Engl. 1960; 27:310–354.16. Kaschten B, Flandroy P, Reznik M, Hainaut H, Stevenaert A. Radiation-induced gliosarcoma. Case report and review of the literature. J Neurosurg. 1995; 83:154–162.17. Kitanaka C, Shitara N, Nakagomi T, et al. Postradiation astrocytoma. Report of two cases. J Neurosurg. 1989; 70:469–474.18. Klériga E, Sher JH, Nallainathan SK, Stein SC, Sacher M. Development of cerebellar malignant astrocytoma at site of a medulloblastoma treated 11 years earlier. Case report. J Neurosurg. 1978; 49:445–449.
Article19. Löning L, Zimmermann M, Reiter A, et al. Secondary neoplasms subsequent to Berlin-Frankfurt-Münster therapy of acute lymphoblastic leukemia in childhood: significantly lower risk without cranial radiotherapy. Blood. 2000; 95:2770–2775.20. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
Article21. Malone M, Lumley H, Erdohazi M. Astrocytoma as a second malignancy in patients with acute lymphoblastic leukemia. Cancer. 1986; 57:1979–1985.
Article22. Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003; 53:524–528.
Article23. Neglia JP, Meadows AT, Robison LL, et al. Second neoplasms after acute lymphoblastic leukemia in childhood. N Engl J Med. 1991; 325:1330–1336.
Article24. Nishio S, Morioka T, Inamura T, et al. Radiation-induced brain tumours: potential late complications of radiation therapy for brain tumours. Acta Neurochir (Wien). 1998; 140:763–770.
Article25. Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005; 109:93–108.
Article26. Okamoto S, Handa H, Yamashita J, Tokuriki Y, Abe M. Post-irradiation brain tumors. Neurol Med Chir (Tokyo). 1985; 25:528–533.
Article27. Park KJ, Kang SH, Lee HG, Chung YG. Olfactory neuroblastoma following treatment for pituitary adenoma. J Neurooncol. 2008; 90:237–241.
Article28. Relling MV, Rubnitz JE, Rivera GK, et al. High incidence of secondary brain tumours after radiotherapy and antimetabolites. Lancet. 1999; 354:34–39.
Article29. Ron E, Modan B, Boice JD Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988; 319:1033–1039.
Article30. Shore RE, Albert RE, Pasternack BS. Follow-up study of patients treated by X-ray epilation for Tinea capitis; resurvey of post-treatment illness and mortality experience. Arch Environ Health. 1976; 31:21–28.31. Soffer D, Gomori JM, Pomeranz S, Siegal T. Gliomas following low-dose irradiation to the head report of three cases. J Neurooncol. 1990; 8:67–72.
Article32. Tanriover N, Ulu MO, Sar M, Uzan M. Anaplastic oligoastrocytoma: previous treatment as a possible cause in a child with acute lymphoblastic leukemia. Childs Nerv Syst. 2007; 23:469–473.
Article33. Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993; 72:2227–2233.
Article34. Walter AW, Hancock ML, Pui CH, et al. Secondary brain tumors in children treated for acute lymphoblastic leukemia at St Jude Children’s Research Hospital. J Clin Oncol. 1998; 16:3761–3767.
Article35. Walters TR. Childhood acute lymphocytic leukemia with a second primary neoplasm. Am J Pediatr Hematol Oncol. 1979; 1:285–287.
Article36. Zhang Y, Goddard K, Spinelli JJ, Gotay C, McBride ML. Risk of late mortality and second malignant neoplasms among 5-Year survivors of young adult cancer: a report of the childhood, adolescent, and young adult cancer survivors research program. J Cancer Epidemiol. 2012; 2012:103032.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Oligodendroglioma after Treatment of Acute Lymphoblastic Leukemia in Childhood
- Meeting Report: 2009 Symposium on Childhood Acute Lymphoblastic Leukemia - Update on the Diagnosis and Treatment for Acute Lymphoblastic Leukemia in Childhood & Adolescence; Seoul; Korea; June 27, 2009
- Advances in the Treatment of Childhood Acute Lymphoblastic Leukemia
- Recent Advance in Treatment of Acute Lymphoblastic Leukemia in Childhood
- Pancreatitis Induced by 6-mercaptopurine and 6-thioguanine in Childhood Acute Lymphoblastic Leukemia