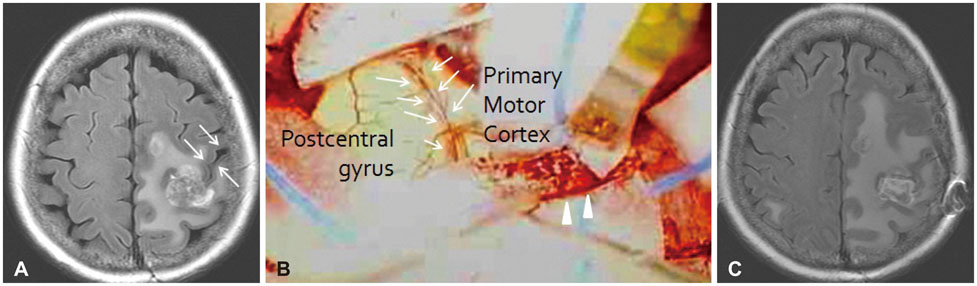
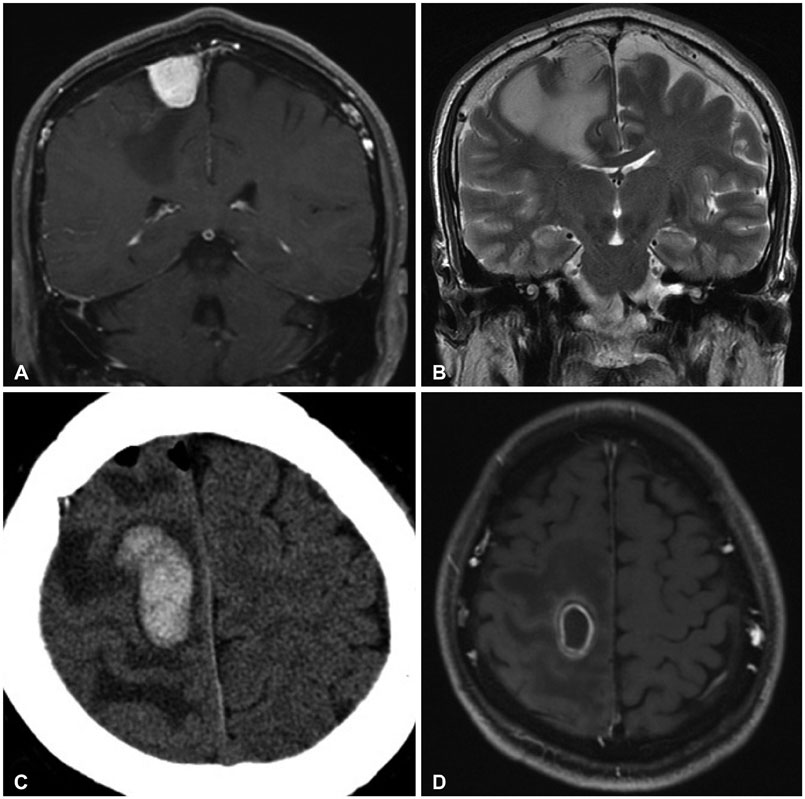
Brain Tumor Res Treat.
2016 Oct;4(2):70-76. 10.14791/btrt.2016.4.2.70.
Surgical Resection of Non-Glial Tumors in the Motor Cortex
- Affiliations
-
- 1Department of Neurosurgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. sunchulh@schmc.ac.kr
- KMID: 2356973
- DOI: http://doi.org/10.14791/btrt.2016.4.2.70
Abstract
- BACKGROUND
Direct surgery to resect tumors in the motor cortex could improve neurological symptoms or cause novel motor weakness. The present study describes the neurological outcomes of patients after the surgical resection of non-glial tumors in the primary motor cortex.
METHODS
The present study included 25 patients who had pathologically confirmed non-glial tumors in the motor cortex for which they underwent surgery. Tumor location was verified using anatomical landmarks on preoperative magnetic resonance imaging scans. All surgeries involved a craniotomy and tumor resection, especially use of the sulcal dissecting approach for intra-axial tumors.
RESULTS
Of the 25 patients, 10 exhibited metastasis, 13 had a meningioma, and 2 had a cavernous malformation. Motor weakness and seizures were the most common symptoms, while 3 patients experienced only a headache. The tumor size was less than 20 mm in 4 patients, 20-40 mm in 14, and greater than 40 mm in seven. Of the 25 patients, 13 exhibited motor weakness prior to the operation, but most of these symptoms (76.9%) improved following surgery. On the other hand, eight patients experienced seizures prior to the surgery, and in three of these patients (37.5%), the seizures were not controlled after the surgery. In terms of surgical complications, a postoperative hematoma developed in one of the meningioma patients, and the patient's hemiparesis was aggravated.
CONCLUSION
The present findings show that careful and meticulous resection of non-glial tumors in the motor cortex can improve preoperative neurological signs, but it cannot completely control seizure activity.
Keyword
MeSH Terms
Figure
Reference
-
1. Ostrý S, Netuka D, Beneš V. Rolandic area meningioma resection controlled and guided by intraoperative cortical mapping. Acta Neurochir (Wien). 2012; 154:843–853.
Article2. Lee JJ, Kim YI, Hong JT, Sung JH, Lee SW, Yang SH. Intraoperative monitoring of motor-evoked potentials for supratentorial tumor surgery. J Korean Neurosurg Soc. 2014; 56:98–102.
Article3. Prabhu SS, Gasco J, Tummala S, Weinberg JS, Rao G. Intraoperative magnetic resonance imaging-guided tractography with integrated monopolar subcortical functional mapping for resection of brain tumors. Clinical article. J Neurosurg. 2011; 114:719–726.
Article4. Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS. Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg. 2015; 11. 6. [Epub]. DOI: 10.3171/2015.5.JNS142833.
Article5. Hervey-Jumper SL, Li J, Lau D, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. J Neurosurg. 2015; 123:325–339.
Article6. Daglioglu E, Ergungor F, Polat E, Nacar O. Microsurgical resection of supratentorial cerebral cavernomas. Turk Neurosurg. 2010; 20:348–352.7. Kim BW, Lee JW, Huh SK, Lee KC. Clinical analysis of microsurgery for brainstem cavernous malformations: surgical indications, optimal approaches, and clinical outcomes. Korean J Cerebrovasc Surg. 2010; 12:169–176.8. Cohen BA, Knopp EA, Rusinek H, Liu S, Gonen O. Brain compression without global neuronal loss in meningiomas: whole-brain proton MR spectroscopy report of 2 cases. AJNR Am J Neuroradiol. 2005; 26:2178–2182.9. Weil RJ, Lonser RR. Selective excision of metastatic brain tumors originating in the motor cortex with preservation of function. J Clin Oncol. 2005; 23:1209–1217.
Article10. Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997; 120(Pt 1):141–157.
Article11. Kido DK, LeMay M, Levinson AW, Benson WE. Computed tomographic localization of the precentral gyrus. Radiology. 1980; 135:373–377.
Article12. Naidich TP, Valavanis AG, Kubik S. Anatomic relationships along the low-middle convexity: Part I--Normal specimens and magnetic resonance imaging. Neurosurgery. 1995; 36:517–532.
Article13. Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009; 110:730–736.
Article14. Stortebecker TP. Metastatic tumors of the brain from a neurosurgical point of view; a follow-up study of 158 cases. J Neurosurg. 1954; 11:84–111.15. Kim SH, Jung S, Kang SS, et al. Evaluation of the postoperative motor function for metastatic brain tumors around the motor cortex. J Korean Neurosurg Soc. 2001; 30:Suppl 1. S25–S29.16. Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood-brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001; 71:188–192.
Article17. Raychaudhuri R, Batjer HH, Awad IA. Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol. 2005; 63:319–328. discussion 328.
Article18. Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev. 1986; 9:177–216.
Article19. McCormick WF. The pathology of vascular ("arteriovenous") malformations. J Neurosurg. 1966; 24:807–816.
Article20. Robinson JR Jr, Awad IA, Magdinec M, Paranandi L. Factors predisposing to clinical disability in patients with cavernous malformations of the brain. Neurosurgery. 1993; 32:730–735. discussion 735-6.
Article21. Zimmerman RS, Spetzler RF, Lee KS, Zabramski JM, Hargraves RW. Cavernous malformations of the brain stem. J Neurosurg. 1991; 75:32–39.
Article22. Kikuta K, Nozaki K, Takahashi JA, Miyamoto S, Kikuchi H, Hashimoto N. Postoperative evaluation of microsurgical resection for cavernous malformations of the brainstem. J Neurosurg. 2004; 101:607–612.
Article23. Commins DL, Atkinson RD, Burnett ME. Review of meningioma histopathology. Neurosurg Focus. 2007; 23:E3.
Article24. International Agency for Research on Cancer. Meningiomas. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4th ed. Geneva: World Health Organization;2007.25. Shinoura N, Suzuki Y, Yamada R, Kodama T, Takahashi M, Yagi K. Restored activation of primary motor area from motor reorganization and improved motor function after brain tumor resection. AJNR Am J Neuroradiol. 2006; 27:1275–1282.26. Suzuki A, Yasui N. Intraoperative localization of the central sulcus by cortical somatosensory evoked potentials in brain tumor. Case report. J Neurosurg. 1992; 76:867–870.
Article27. Cedzich C, Taniguchi M, Schäfer S, Schramm J. Somatosensory evoked potential phase reversal and direct motor cortex stimulation during surgery in and around the central region. Neurosurgery. 1996; 38:962–970.
Article28. Tamura M, Muragaki Y, Saito T. Strategy of surgical resection for glioma based on intraoperative functional mapping and monitoring. Neurol Med Chir (Tokyo). 2015; 55:383–398.
Article29. Krieg SM, Schäffner M, Shiban E, et al. Reliability of intraoperative neurophysiological monitoring using motor evoked potentials during resection of metastases in motor-eloquent brain regions: clinical article. J Neurosurg. 2013; 118:1269–1278.
Article30. Hardwidge C, Hettige S. Tumours of the central nervous system. Surgery. 2012; 30:155–161.
Article31. Duffau H. Awake mapping of the brain connectome in glioma surgery: concept is stronger than technology. Eur J Surg Oncol. 2015; 41:1261–1263.
Article32. Duffau H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol. 2015; 11:255–265.
Article33. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007; 6:421–430.
Article34. Englot DJ, Han SJ, Berger MS, Barbaro NM, Chang EF. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012; 70:921–928. discussion 928.
Article35. Mittal S, Barkmeier D, Hua J. Intracranial EEG analysis in tumor-related epilepsy: evidence of distant epileptic abnormalities. Clin Neurophysiol. 2016; 127:238–244.
Article36. Seo DW, Hong SB. Epileptogenic foci on subdural recording in intractable epilepsy patients with temporal dysembryoplastic neuroepithelial tumor. J Korean Med Sci. 2003; 18:559–565.
Article37. Fish DR, Spencer SS. Clinical correlations: MRI and EEG. Magn Reson Imaging. 1995; 13:1113–1117.
Article38. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008; 7:525–537.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic stimulation of the motor cortex and motor root in cervicalspondylosis
- Anatomicophysiologic Monitoring in the Surgery of Central Area
- Magnetic stimulation of motor cortex and spinal motor root
- Epidural Motor Cortex Stimulation for Intractable Thalamic Pain: A case report
- Limitation of Intraoperative Transcranial Electrical Stimulation-Motor Evoked Potential Monitoring During Brain Tumor Resection Adjacent to the Primary Motor Cortex