J Periodontal Implant Sci.
2015 Dec;45(6):229-237. 10.5051/jpis.2015.45.6.229.
Assessment of dehydrothermally cross-linked collagen membrane for guided bone regeneration around peri-implant dehiscence defects: a randomized single-blinded clinical trial
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. drjew@yuhs.ac
- 2Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea.
- KMID: 2354941
- DOI: http://doi.org/10.5051/jpis.2015.45.6.229
Abstract
- PURPOSE
The aim of this study was to determine the clinical feasibility of using dehydrothermally cross-linked collagen membrane (DCM) for bone regeneration around peri-implant dehiscence defects, and compare it with non-cross-linked native collagen membrane (NCM).
METHODS
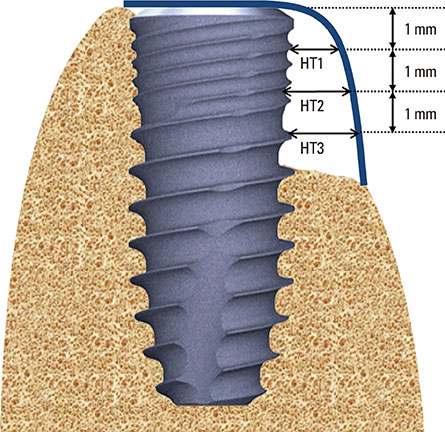
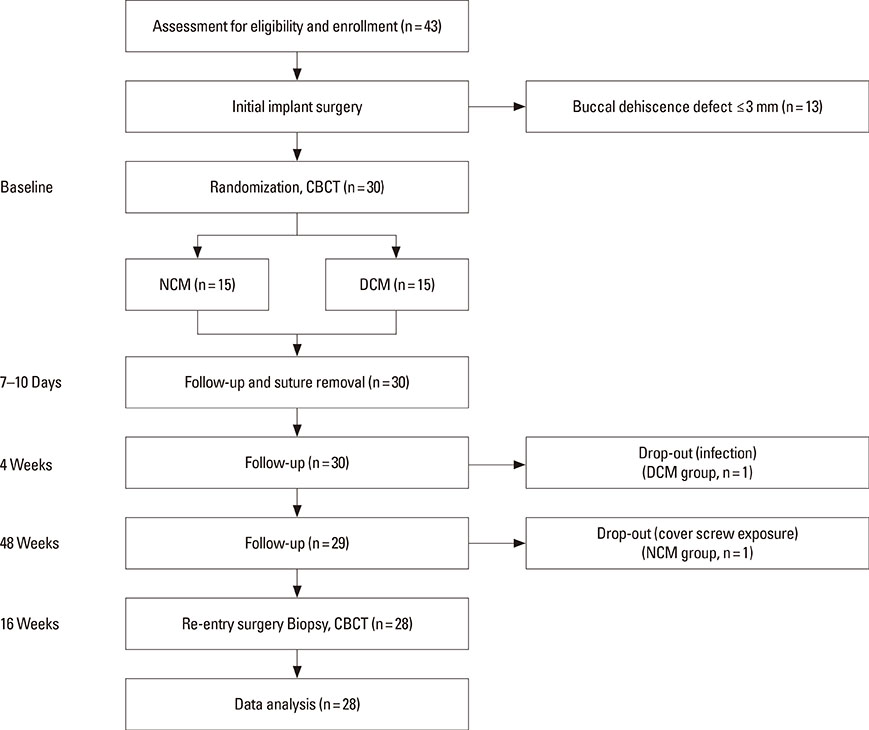
Dehiscence defects were investigated in twenty-eight patients. Defect width and height were measured by periodontal probe immediately following implant placement (baseline) and 16 weeks afterward. Membrane manipulation and maintenance were clinically assessed by means of the visual analogue scale score at baseline. Changes in horizontal thickness at 1 mm, 2 mm, and 3 mm below the top of the implant platform and the average bone density were assessed by cone-beam computed tomography at 16 weeks. Degradation of membrane was histologically observed in the soft tissue around the implant prior to re-entry surgery.
RESULTS
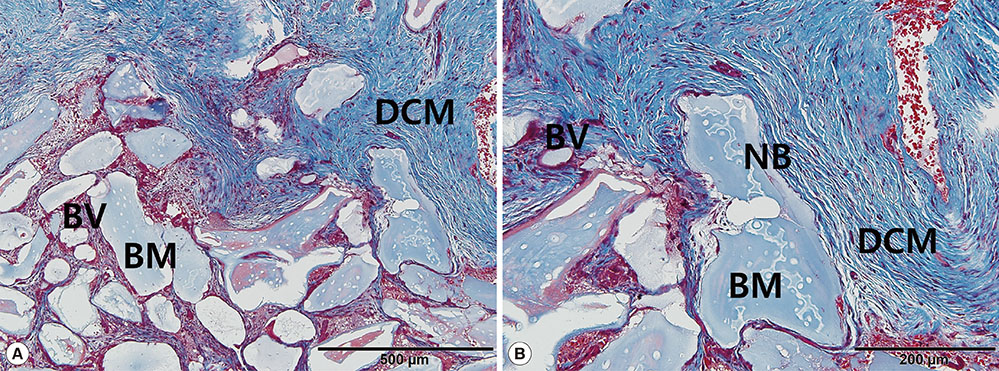
Five defect sites (two sites in the NCM group and three sites in the DCM group) showed soft-tissue dehiscence defects and membrane exposure during the early healing period, but there were no symptoms or signs of severe complications during the experimental postoperative period. Significant clinical and radiological improvements were found in all parameters with both types of collagen membrane. Partially resorbed membrane leaflets were only observed histologically in the DCM group.
CONCLUSIONS
These findings suggest that, compared with NCM, DCM has a similar clinical expediency and possesses more stable maintenance properties. Therefore, it could be used effectively in guided bone regeneration around dehiscence-type defects.
MeSH Terms
Figure
Cited by 1 articles
-
Dental alloplastic bone substitutes currently available in Korea
Jeong-Kui Ku, Inseok Hong, Bu-Kyu Lee, Pil-Young Yun, Jeong Keun Lee
J Korean Assoc Oral Maxillofac Surg. 2019;45(2):51-67. doi: 10.5125/jkaoms.2019.45.2.51.
Reference
-
1. Chiapasco M, Zaniboni M. Clinical outcomes of GBR procedures to correct peri-implant dehiscences and fenestrations: a systematic review. Clin Oral Implants Res. 2009; 20:Suppl 4. 113–136.
Article2. Retzepi M, Donos N. Guided Bone Regeneration: biological principle and therapeutic applications. Clin Oral Implants Res. 2010; 21:567–643.
Article3. Locci P, Calvitti M, Belcastro S, Pugliese M, Guerra M, Marinucci L, et al. Phenotype expression of gingival fibroblasts cultured on membranes used in guided tissue regeneration. J Periodontol. 1997; 68:857–920.
Article4. Schwarz F, Rothamel D, Herten M, Wüstefeld M, Sager M, Ferrari D, et al. Immunohistochemical characterization of guided bone regeneration at a dehiscence-type defect using different barrier membranes: an experimental study in dogs. Clin Oral Implants Res. 2008; 19:402–417.
Article5. Behring J, Junker R, Walboomers XF, Chessnut B, Jansen JA. Toward guided tissue and bone regeneration: morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology. 2008; 96:1–11.
Article6. Schwarz F, Rothamel D, Herten M, Sager M, Becker J. Angiogenesis pattern of native and cross-linked collagen membranes: an immunohistochemical study in the rat. Clin Oral Implants Res. 2006; 17:403–412.
Article7. Sela MN, Kohavi D, Krausz E, Steinberg D, Rosen G. Enzymatic degradation of collagen-guided tissue regeneration membranes by periodontal bacteria. Clin Oral Implants Res. 2003; 14:263–271.
Article8. Jung RE, Fenner N, Hämmerle CH, Zitzmann NU. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12-14 years. Clin Oral Implants Res. 2013; 24:1065–1138.9. Drexler JW, Powell HM. Dehydrothermal crosslinking of electrospun collagen. Tissue Eng Part C Methods. 2011; 17:9–17.
Article10. Schwarz F, Sager M, Rothamel D, Herten M, Sculean A, Becker J. Use of native and cross-linked collagen membranes for guided tissue and bone regeneration. Schweiz Monatsschr Zahnmed. 2006; 116:1112–1135.11. Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003; 24:759–826.
Article12. Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996; 17:765–838.
Article13. Zahedi S, Bozon C, Brunel G. A 2-year clinical evaluation of a diphenylphosphorylazide-cross-linked collagen membrane for the treatment of buccal gingival recession. J Periodontol. 1998; 69:975–1056.
Article14. Becker J, Al-Nawas B, Klein MO, Schliephake H, Terheyden H, Schwarz F. Use of a new cross-linked collagen membrane for the treatment of dehiscence-type defects at titanium implants: a prospective, randomized-controlled double-blinded clinical multicenter study. Clin Oral Implants Res. 2009; 20:742–751.
Article15. Annen BM, Ramel CF, Hämmerle CH, Jung RE. Use of a new cross-linked collagen membrane for the treatment of peri-implant dehiscence defects: a randomised controlled double-blinded clinical trial. Eur J Oral Implantology. 2011; 4:87–100.16. Marzec E, Pietrucha K. The effect of different methods of cross-linking of collagen on its dielectric properties. Biophys Chem. 2008; 132:89–96.
Article17. Haugh MG, Jaasma MJ, O’Brien FJ. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J Biomed Mater Res A. 2009; 89:363–372.
Article18. Rothamel D, Benner M, Fienitz T, Happe A, Kreppel M, Nickenig HJ, et al. Biodegradation pattern and tissue integration of native and cross-linked porcine collagen soft tissue augmentation matrices - an experimental study in the rat. Head Face Med. 2014; 10:10.
Article19. Morris K. Revising the Declaration of Helsinki. Lancet. 2013; 381:1889–1979.
Article20. Stoecklin-Wasmer C, Rutjes AW, da Costa BR, Salvi GE, Jüni P, Sculean A. Absorbable collagen membranes for periodontal regeneration: a systematic review. J Dent Res. 2013; 92:773–854.
Article21. Gholami GA, Najafi B, Mashhadiabbas F, Goetz W, Najafi S. Clinical, histologic and histomorphometric evaluation of socket preservation using a synthetic nanocrystalline hydroxyapatite in comparison with a bovine xenograft: a randomized clinical trial. Clin Oral Implants Res. 2012; 23:1198–1402.
Article22. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009; 41:1149–1209.
Article23. Lorenzoni M, Pertl C, Polansky RA, Jakse N, Wegscheider WA. Evaluation of implants placed with barrier membranes. A restrospective follow-up study up to five years. Clin Oral Implants Res. 2002; 13:274–354.24. Donos N, Kostopoulos L, Karring T. Alveolar ridge augmentation using a resorbable copolymer membrane and autogenous bone grafts. An experimental study in the rat. Clin Oral Implants Res. 2002; 13:203–216.25. Moses O, Pitaru S, Artzi Z, Nemcovsky CE. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005; 16:210–219.
Article26. Friedmann A, Strietzel FP, Maretzki B, Pitaru S, Bernimoulin JP. Observations on a new collagen barrier membrane in 16 consecutively treated patients. Clinical and histological findings. J Periodontol. 2001; 72: 1616–1639.
Article27. Turkyilmaz I, McGlumphy EA. Influence of bone density on implant stability parameters and implant success: a retrospective clinical study. BMC Oral Health. 2008; 8:32.
Article28. Owens KW, Yukna RA. Collagen membrane resorption in dogs: a comparative study. Implant Dent. 2001; 10:49–58.
Article29. Rothamel D, Schwarz F, Sculean A, Herten M, Scherbaum W, Becker J. Biocompatibility of various collagen membranes in cultures of human PDL fibroblasts and human osteoblast-like cells. Clin Oral Implants Res. 2004; 15:443–452.
Article30. Veríssimo DM, Leitão RF, Ribeiro RA, Figueiró SD, Sombra AS, Góes JC, et al. Polyanionic collagen membranes for guided tissue regeneration: Effect of progressive glutaraldehyde cross-linking on biocompatibility and degradation. Acta Biomater. 2010; 6:4011–4019.
Article31. Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater. 2009; 2:202–211.
Article32. Weadock KS, Miller EJ, Bellincampi LD, Zawadsky JP, Dunn MG. Physical crosslinking of collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res. 1995; 29:1373–1382.
Article33. Cornwell KG, Lei P, Andreadis ST, Pins GD. Crosslinking of discrete self-assembled collagen threads: Effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res A. 2007; 80:362–433.
Article34. Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng Part B Rev. 2015; 21:298–313.
Article35. Junker R, Dimakis A, Thoneick M, Jansen JA. Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:Suppl 4. 185–206.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of clinical and radiographic outcomes of guided bone regeneration with dehydrothermally cross-linked collagen membrane around peri-implant dehiscence defects: Results from a 3-year randomized clinical trial
- Guided bone regeneration in peri-implant defects using a 1:1 mixture of cancellous and cortical freeze-dried bone allograft: A randomized controlled trial
- Clinical and histopathological study on the effect of Nonresorbable membrane with Demineralized freeze dried bone graft for Guided Bone Regeneration in Implant Dehiscence Defects
- Comparison of Resorbable and Nonresorbable Membrane for Guided Bone Regeneration in Implant Dehiscence Defects
- The Effect of Demineralized Freeze-Dried Bone Allograft in Guided Bone Regeneration on Supra-Alveolar Peri-Implant Defects in Dogs