J Clin Neurol.
2016 Apr;12(2):129-136. 10.3988/jcn.2016.12.2.129.
Considerations When Subtyping Ischemic Stroke in Asian Patients
- Affiliations
-
- 1Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ohyoung.bang@samsung.com
- KMID: 2354131
- DOI: http://doi.org/10.3988/jcn.2016.12.2.129
Abstract
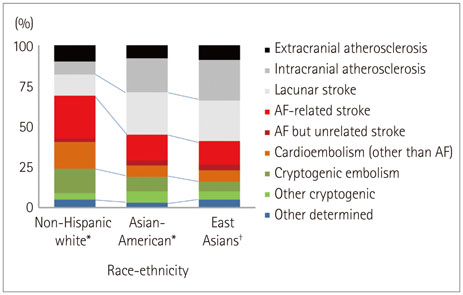
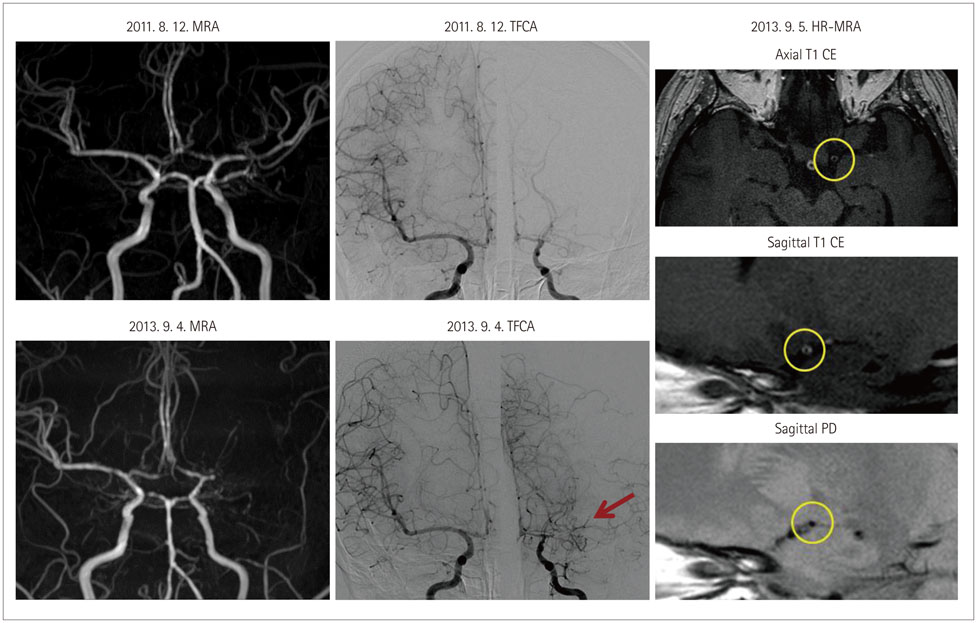
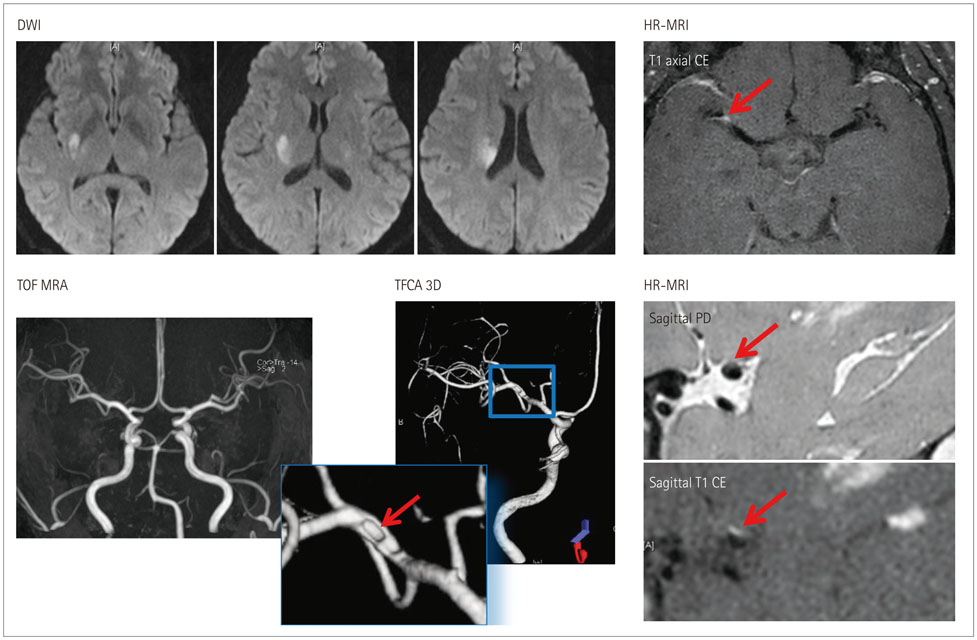
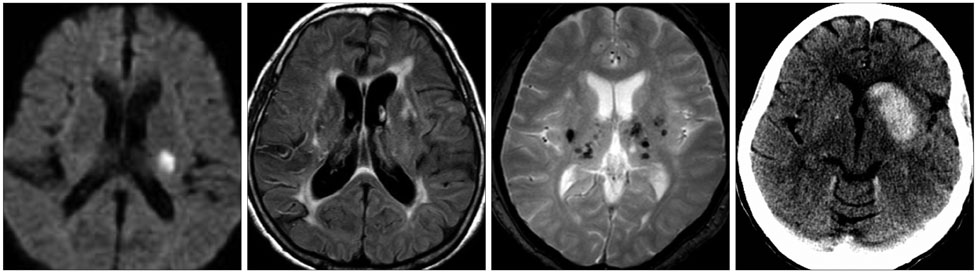
- Both the incidence and prevalence of stroke in Asia are steadily increasing, and the burden of stroke is particularly high in Asian countries. Although strokes in Asians and Caucasians share many common features, there are some differences that are probably due to differences in lifestyle and genetic background. While there have been advances in the stroke classification system, the assignment of Asian stroke patients to etiological categories has received little attention. The current classification system may not be well suited to Asian patients with ischemic stroke because the proportions and relative importance of stroke subtypes may differ with race and ethnicity. This review addresses concerns about the use of the current stroke classification system in Asian patients with ischemic stroke, and proposes a classification system that is more specific to the Asian population, in conjunction with discussing advances in diagnostic techniques.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Association between Stroke Status and Depression in a Community Setting: The 2014 Korea National Health and Nutrition Examination Survey
Mina Kim, Gyung-Jae Oh, Young-Hoon Lee
J Clin Neurol. 2017;13(1):55-61. doi: 10.3988/jcn.2017.13.1.55.Cerebral Arterial Stenosis in Patients with Spontaneous Intracerebral Hemorrhage
Pil-Wook Chung, Yu Sam Won
J Korean Neurosurg Soc. 2017;60(5):511-517. doi: 10.3340/jkns.2016.1011.003.
Reference
-
1. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991; 337:1521–1526.
Article2. Gross CR, Shinar D, Mohr JP, Hier DB, Caplan LR, Price TR, et al. Interobserver agreement in the diagnosis of stroke type. Arch Neurol. 1986; 43:893–898.
Article3. Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988; 19:1083–1092.
Article4. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
Article5. Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol. 2005; 58:688–697.
Article6. Amarenco P, Bogousslavsky J, Caplan LR, Donnan GA, Hennerici MG. New approach to stroke subtyping: the A-S-C-O (phenotypic) classification of stroke. Cerebrovasc Dis. 2009; 27:502–508.
Article7. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013; 1:e259–e281.
Article8. Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci. 2009; 284:40–45.
Article9. White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005; 111:1327–1331.
Article10. Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. 2014; 16:8–17.
Article11. Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry. 2014; 85:1308–1312.
Article12. Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011; 124:314–323.
Article13. Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008; 39:2396–2399.14. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006; 1:158–159.
Article15. Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995; 45:659–663.
Article16. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013; 34:299–304.
Article17. Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. 2012; 71:195–198.
Article18. Majidi S, Sein J, Watanabe M, Hassan AE, Van de Moortele PF, Suri MF, et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol. 2013; 34:2259–2264.
Article19. Kamada F, Aoki Y, Narisawa A, Abe Y, Komatsuzaki S, Kikuchi A, et al. A genome-wide association study identifies RNF213 as the first Moyamoya disease gene. J Hum Genet. 2011; 56:34–40.
Article20. Liu W, Morito D, Takashima S, Mineharu Y, Kobayashi H, Hitomi T, et al. Identification of RNF213 as a susceptibility gene for moyamoya disease and its possible role in vascular development. PLoS One. 2011; 6:e22542.
Article21. Fujimura M, Sonobe S, Nishijima Y, Niizuma K, Sakata H, Kure S, et al. Genetics and biomarkers of Moyamoya disease: significance of RNF213 as a susceptibility gene. J Stroke. 2014; 16:65–72.
Article22. Liu W, Hashikata H, Inoue K, Matsuura N, Mineharu Y, Kobayashi H, et al. A rare Asian founder polymorphism of Raptor may explain the high prevalence of Moyamoya disease among East Asians and its low prevalence among Caucasians. Environ Health Prev Med. 2010; 15:94–104.
Article23. Liu W, Hitomi T, Kobayashi H, Harada KH, Koizumi A. Distribution of moyamoya disease susceptibility polymorphism p.R4810K in RNF213 in East and Southeast Asian populations. Neurol Med Chir (Tokyo). 2012; 52:299–303.
Article24. Bang OY, Lee PH, Yoon SR, Lee MA, Joo IS, Huh K. Inflammatory markers, rather than conventional risk factors, are different between carotid and MCA atherosclerosis. J Neurol Neurosurg Psychiatry. 2005; 76:1128–1134.
Article25. Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS, et al. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005; 65:296–298.
Article26. López-Cancio E, Galán A, Dorado L, Jiménez M, Hernández M, Millán M, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. 2012; 43:2712–2719.
Article27. Akins PT, Pilgram TK, Cross DT 3rd, Moran CJ. Natural history of stenosis from intracranial atherosclerosis by serial angiography. Stroke. 1998; 29:433–438.
Article28. Ryoo S, Park JH, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Branch occlusive disease: clinical and magnetic resonance angiography findings. Neurology. 2012; 78:888–896.
Article29. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. 2014; 16:27–35.
Article30. Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008; 39:1142–1147.
Article31. Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. 2011; 42:2957–2959.
Article32. Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: analysis of indicators for small vessel disease and atherosclerosis. Stroke. 2010; 41:2822–2827.
Article33. Turan TN, Derdeyn CP, Fiorella D, Chimowitz MI. Treatment of atherosclerotic intracranial arterial stenosis. Stroke. 2009; 40:2257–2261.
Article34. Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005; 128(Pt 11):2507–2517.
Article35. Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011; 82:126–135.
Article36. Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013; 44:995–1001.37. Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008; 39:1414–1420.
Article38. Conklin J, Silver FL, Mikulis DJ, Mandell DM. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014; 76:899–904.
Article39. Park JH, Ryoo S, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Differential risk factors for lacunar stroke depending on the MRI (white and red) subtypes of microangiopathy. PLoS One. 2012; 7:e44865.
Article40. Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012; 43:2916–2922.
Article41. Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015; 84:825–832.
Article42. Vernooij MW, Haag MD, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009; 66:714–720.
Article43. Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008; 255:1679–1686.
Article44. Kim SJ, Ryoo S, Kwon S, Park YK, Kim JP, Lee GY, et al. Is atrial fibrillation always a culprit of stroke in patients with atrial fibrillation plus stroke? Cerebrovasc Dis. 2013; 36:373–382.
Article45. Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke. 2014; 45:1186–1194.
Article46. Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014; 45:2457–2460.
Article47. Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, et al. High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol. 2013; 20:1311–1318.
Article48. Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. 2015; 46:697–703.
Article49. Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015; 14:640–654.
Article50. Bang OY, Ryoo S, Kim SJ, Yoon CH, Cha J, Yeon JY, et al. Adult Moyamoya disease: a burden of intracranial stenosis in East Asians? PLoS One. 2015; 10:e0130663.
Article51. Wysokinski WE, Ammash N, Sobande F, Kalsi H, Hodge D, McBane RD. Predicting left atrial thrombi in atrial fibrillation. Am Heart J. 2010; 159:665–671.
Article52. Okuyama H, Hirono O, Liu L, Takeishi Y, Kayama T, Kubota I. Higher levels of serum fibrin-monomer reflect hypercoagulable state and thrombus formation in the left atrial appendage in patients with acute ischemic stroke. Circ J. 2006; 70:971–976.
Article53. Rodríguez-Yáñez M, Arias-Rivas S, Santamaría-Cadavid M, Sobrino T, Castillo J, Blanco M. High pro-BNP levels predict the occurrence of atrial fibrillation after cryptogenic stroke. Neurology. 2013; 81:444–447.
Article54. Jickling GC, Stamova B, Ander BP, Zhan X, Liu D, Sison SM, et al. Prediction of cardioembolic, arterial, and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke. 2012; 43:2036–2041.
Article55. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012; 60:531–538.
Article56. Lee JM, Shim J, Uhm JS, Kim YJ, Lee HJ, Pak HN, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol. 2014; 113:963–969.
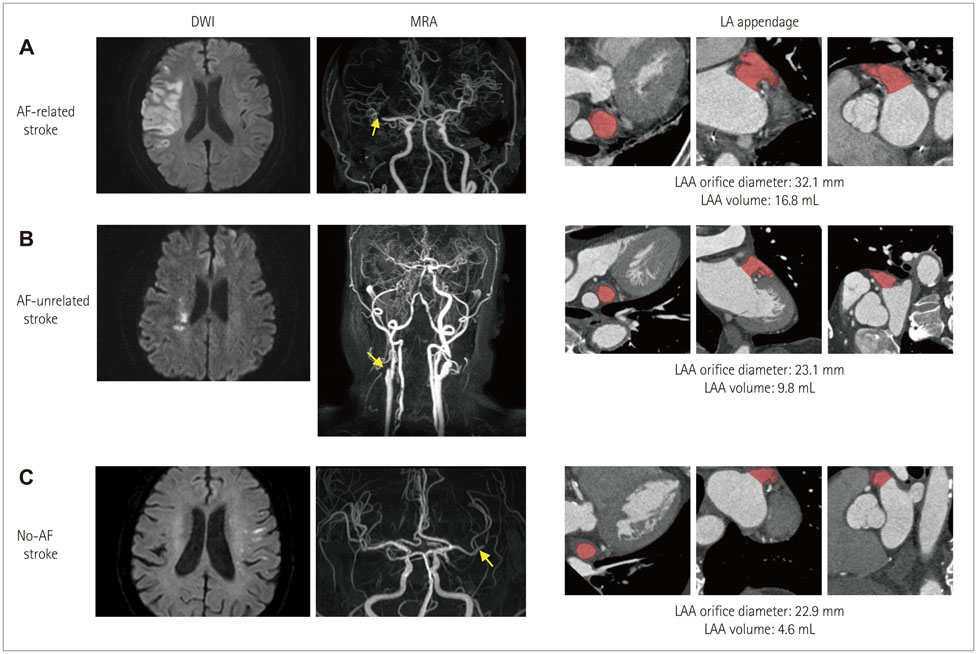
Article57. Jeong WK, Choi JH, Son JP, Lee S, Lee MJ, Choe YH, et al. Volume and morphology of left atrial appendage as determinants of stroke subtype in patients with atrial fibrillation. Heart Rhythm. 2015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack
- Risk Factors and Biomarkers of Ischemic Stroke in Cancer Patients
- Diagnosis of Cerebrovascular Disease
- The Migraine-Stroke Connection
- Simultaneous Onset of Ischemic and Hemorrhagic Stroke Due To Intracranial Artery Dissection