J Clin Neurol.
2016 Jul;12(3):262-273. 10.3988/jcn.2016.12.3.262.
Intraoperative Monitoring and Mapping of the Functional Integrity of the Brainstem
- Affiliations
-
- 1Laboratory for Human and Experimental Neurophysiology, School of Medicine, Split, Croatia. vdeletis@chpnet.org
- 2Department of Intraoperative Neurophysiology, University Hospital of Bellvitge, Barcelona, Spain.
- KMID: 2354112
- DOI: http://doi.org/10.3988/jcn.2016.12.3.262
Abstract
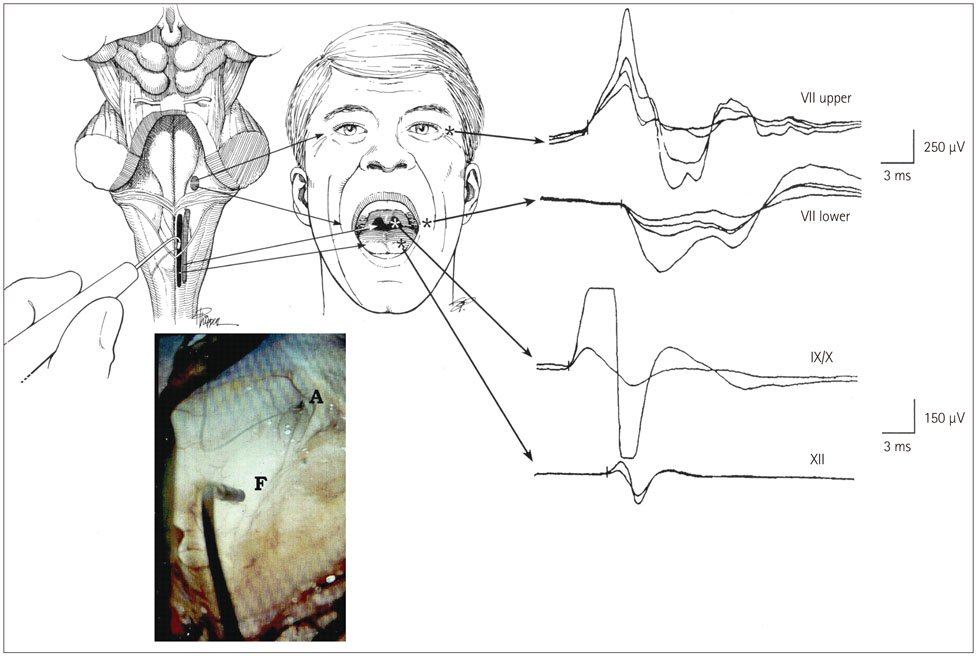
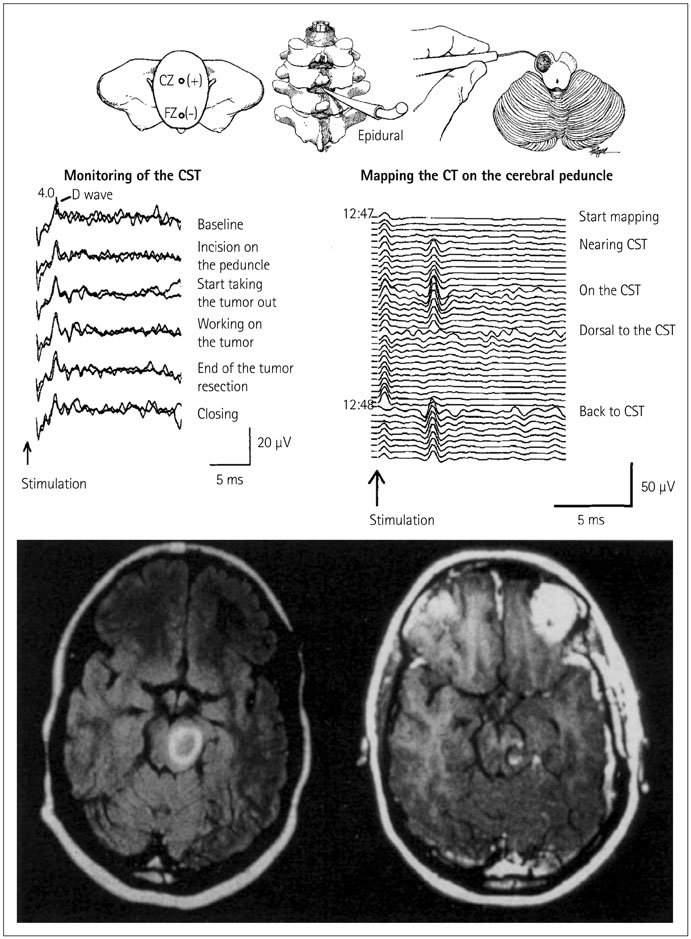
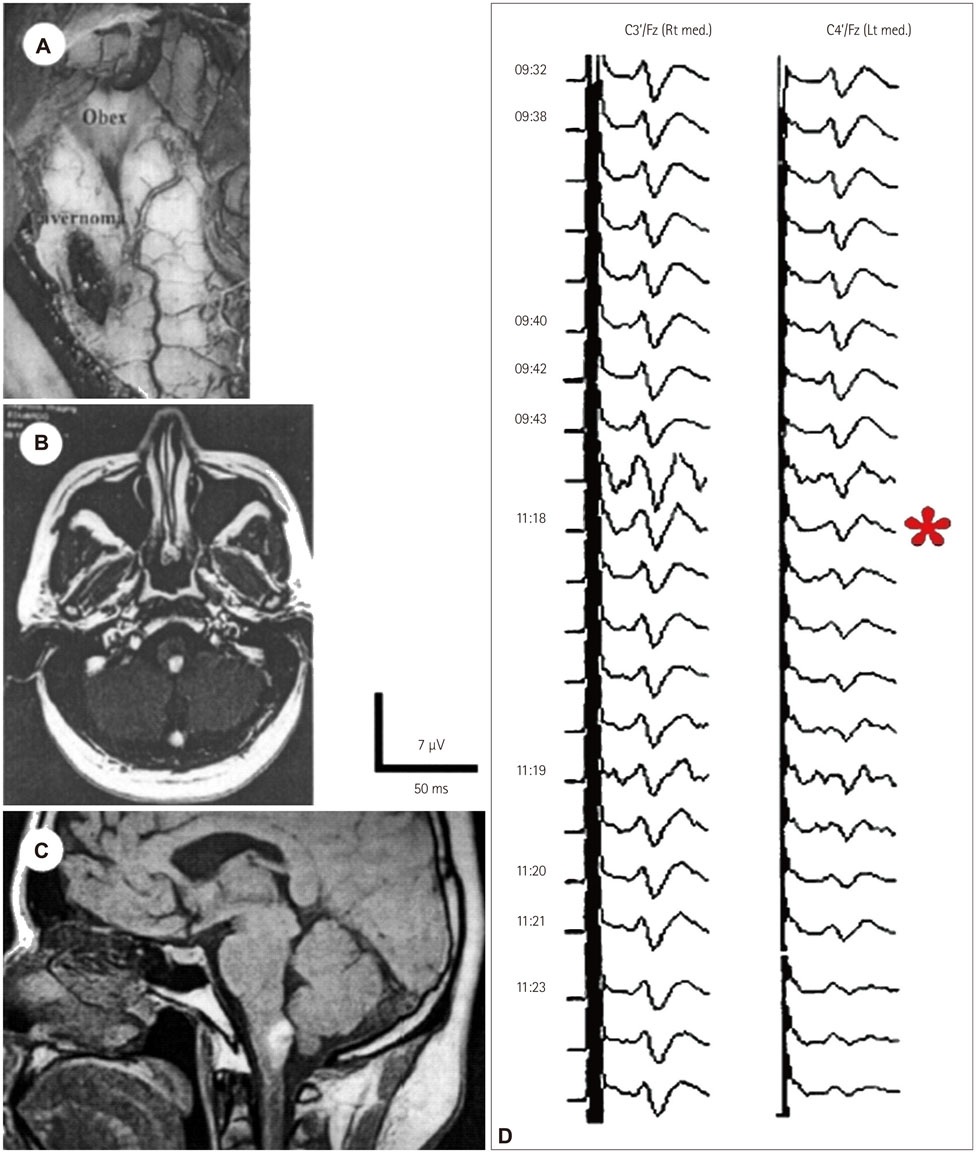
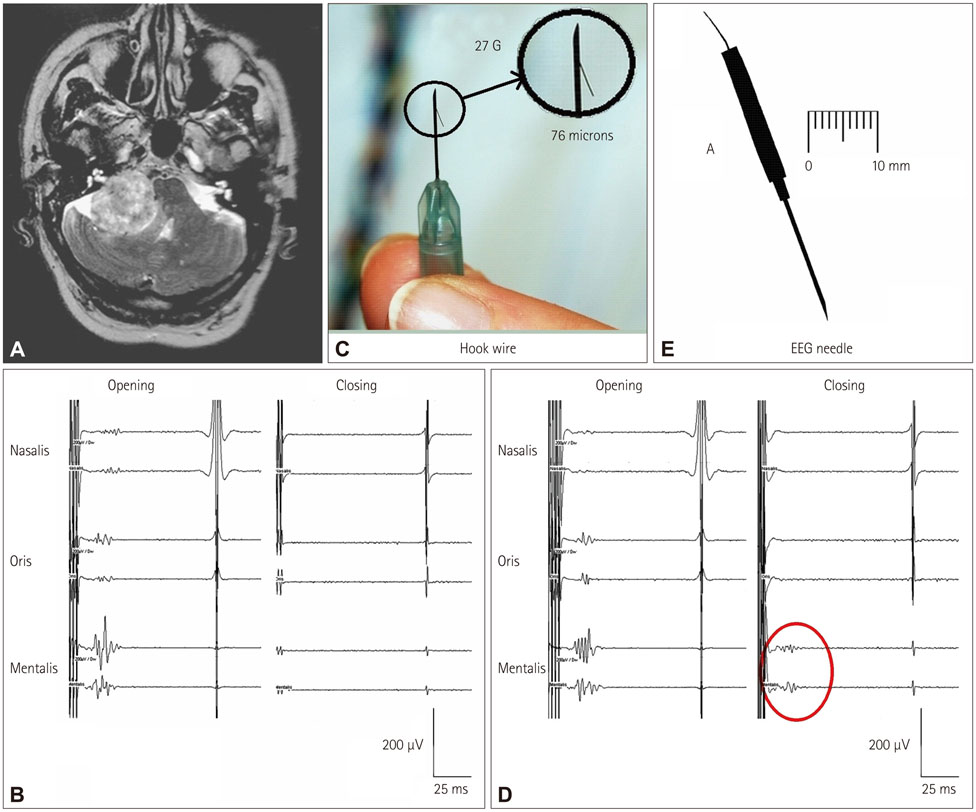
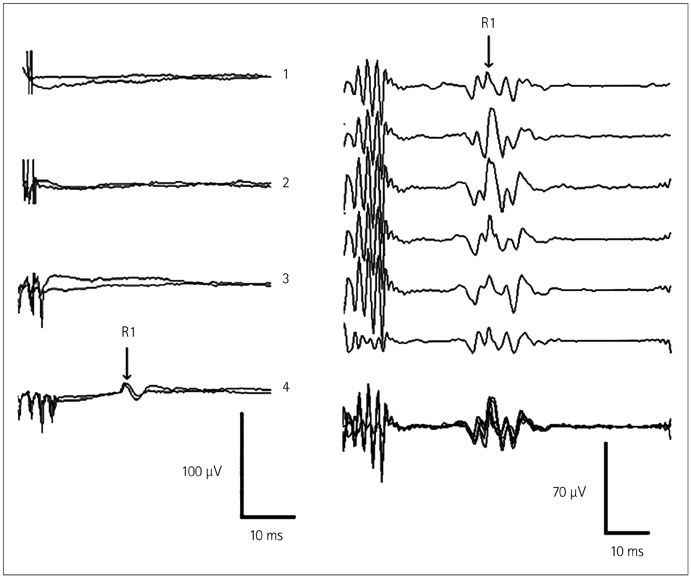
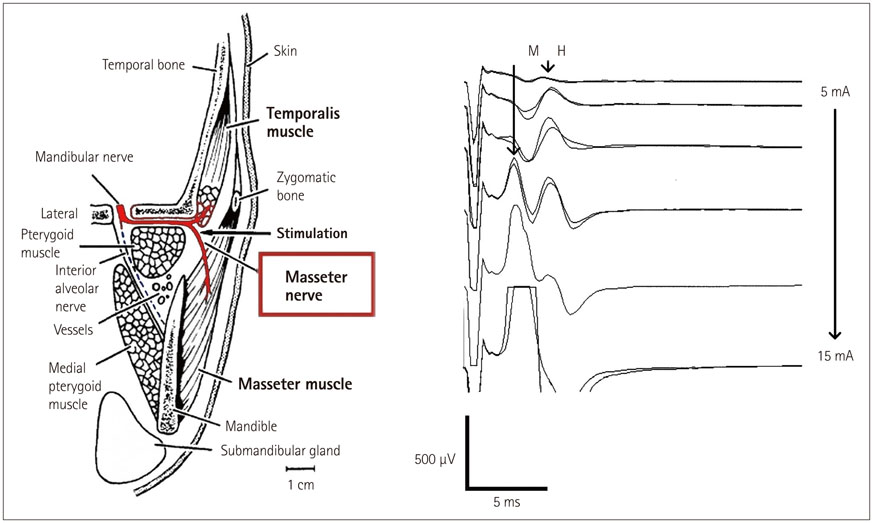
- The risk of iatrogenic damage is very high in surgical interventions in or around the brainstem. However, surgical techniques and intraoperative neuromonitoring (ION) have evolved sufficiently to increase the likelihood of successful functional outcomes in many patients. We present a critical review of the methodologies available for intraoperative monitoring and mapping of the brainstem. There are three main groups of techniques that can be used to assess the functional integrity of the brainstem: 1) mapping, which provides rapid anatomical identification of neural structures using electrical stimulation with a hand-held probe, 2) monitoring, which provides real-time information about the functional integrity of the nervous tissue, and 3) techniques involving the examination of brainstem reflexes in the operating room, which allows for the evaluation of the reflex responses that are known to be crucial for most brainstem functions. These include the blink reflex, which is already in use, and other brainstem reflexes that are being explored, such as the masseter H-reflex. This is still under development but is likely to have important functional consequences. Today an abundant armory of ION methods is available for the monitoring and mapping of the functional integrity of the brainstem during surgery. ION methods are essential in surgery either in or around the brainstem; they facilitate the removal of lesions and contribute to notable improvements in the functional outcomes of patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Neuloh G, Bogucki J, Schramm J. Intraoperative preservation of corticospinal function in the brainstem. J Neurol Neurosurg Psychiatry. 2009; 80:417–422.
Article2. Dong CC, Macdonald DB, Akagami R, Westerberg B, Alkhani A, Kanaan I, et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005; 116:588–596.
Article3. Deletis V, Fernandez-Conejero I, Ulkatan S, Costantino P. Methodology for intraoperatively eliciting motor evoked potentials in the vocal muscles by electrical stimulation of the corticobulbar tract. Clin Neurophysiol. 2009; 120:336–341.
Article4. Deletis V, Fernández-Conejero I, Ulkatan S, Rogić M, Carbó EL, Hiltzik D. Methodology for intra-operative recording of the corticobulbar motor evoked potentials from cricothyroid muscles. Clin Neurophysiol. 2011; 122:1883–1889.
Article5. Strauss C, Romstöck J, Nimsky C, Fahlbusch R. Intraoperative identification of motor areas of the rhomboid fossa using direct stimulation. J Neurosurg. 1993; 79:393–399.
Article6. Morota N, Deletis V. The importance of brainstem mapping in brainstem surgical anatomy before the fourth ventricle and implication for intraoperative neurophysiological mapping. Acta Neurochir (Wien). 2006; 148:499–509. discussion 509.
Article7. Deletis V. Evoked Potentials. In : Lake CL, editor. Clinical Monitoring for Anesthesia and Critical Care. 2nd ed. Lake Carol: W.B. Saunders;1994. p. 282–314.8. Morota N, Deletis V, Lee M, Epstein FJ. Functional anatomic relationship between brain-stem tumors and cranial motor nuclei. Neurosurgery. 1996; 39:787–793. discussion 793-794.
Article9. Liscić RM, Morota N, Deletis V. Intramedullar stimulation of the facial and hypoglossal nerves: estimation of the stimulated site. Croat Med J. 2000; 41:384–388.10. Morota N, Deletis V, Epstein FJ, Kofler M, Abbott R, Lee M, et al. Brain stem mapping: neurophysiological localization of motor nuclei on the floor of the fourth ventricle. Neurosurgery. 1995; 37:922–929. discussion 929-930.11. Romstöck J, Strauss C, Fahlbusch R. Identification of cranial nerve nuclei. Muscle Nerve. 2000; 23:1445–1446.
Article12. Romstöck J, Strauss C, Fahlbusch R. Continuous electromyography monitoring of motor cranial nerves during cerebellopontine angle surgery. J Neurosurg. 2000; 93:586–593.
Article13. Prell J, Strauss C, Rachinger J, Scheller C, Alfieri A, Herfurth K, et al. The intermedius nerve as a confounding variable for monitoring of the free-running electromyogram. Clin Neurophysiol. 2015; 126:1833–1839.
Article14. Deletis V, Sala F, Morota N. Intraoperative neurophysiological monitoring and mapping during brain stem surgery: a modern approach. Oper Tech Neurosurg. 2000; 2:109–113.
Article15. Fahlbusch R, Strauss C. [Surgical significance of cavernous hemangioma of the brain stem]. Zentralbl Neurochir. 1991; 52:25–32.16. Polo G, Fischer C, Sindou MP, Marneffe V. Brainstem auditory evoked potential monitoring during microvascular decompression for hemifacial spasm: intraoperative brainstem auditory evoked potential changes and warning values to prevent hearing loss--prospective study in a consecutive series of 84 patients. Neurosurgery. 2004; 54:97–104. discussion 104-106.
Article17. Quiñones-Hinojosa A, Alam M, Lyon R, Yingling CD, Lawton MT. Transcranial motor evoked potentials during basilar artery aneurysm surgery: technique application for 30 consecutive patients. Neurosurgery. 2004; 54:916–924. discussion 924.
Article18. Macdonald DB, Skinner S, Shils J, Yingling C. American Society of Neurophysiological Monitoring. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013; 124:2291–2316.
Article19. Verst SM, Sucena AC, Maldaun MV, Aguiar PH. Effectiveness of C5 or C6-Cz assembly in predicting immediate post operative facial nerve deficit. Acta Neurochir (Wien). 2013; 155:1863–1869.
Article20. Ulkatan S, Deletis V, Fernandez-Conejero I. Central or peripheral activations of the facial nerve? J Neurosurg. 2007; 106:519–520. author reply 520.
Article21. Fernández-Conejero I, Deletis V. Transcranial electrical stimulation and monitoring. J Neurosurg. 2014; 120:291–292.22. Bostock H, Lin CS, Howells J, Trevillion L, Jankelowitz S, Burke D. After-effects of near-threshold stimulation in single human motor axons. J Physiol. 2005; 564(Pt 3):931–940.
Article23. Fernández-Conejero I, Ulkatan S, Sen C, Deletis V. Intra-operative neurophysiology during microvascular decompression for hemifacial spasm. Clin Neurophysiol. 2012; 123:78–83.
Article24. Malcharek MJ, Landgraf J, Hennig G, Sorge O, Aschermann J, Sablotzki A. Recordings of long-latency trigeminal somatosensoryevoked potentials in patients under general anaesthesia. Clin Neurophysiol. 2011; 122:1048–1054.
Article25. Deletis V, Urriza J, Ulkatan S, Fernandez-Conejero I, Lesser J, Misita D. The feasibility of recording blink reflexes under general anesthesia. Muscle Nerve. 2009; 39:642–646.
Article26. Godaux E, Desmedt JE. Exteroceptive suppression and motor control of the masseter and temporalis muscles in normal man. Brain Res. 1975; 85:447–458.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advancing Intraoperative Neurophysiological Monitoring With Human Reflexes
- Interruption of bispectral index monitoring by nerve integrity monitoring during tympanoplasty: A case report
- The Comparison of the Anesthetic Regimens for Functional Direct Cortical Stimulation Mapping during Craniotomy
- Microvascular Decompression for Tinnitus
- Surgery for seizure-related structural lesions of the brain with intraoperative acute recording(ECoG) and functional mapping