Allergy Asthma Immunol Res.
2016 Nov;8(6):522-526. 10.4168/aair.2016.8.6.522.
Plasma Levels of Matrix Metalloproteinase-9 in Children With Chronic Spontaneous Urticaria
- Affiliations
-
- 1Department of Pediatric Allergy and Immunology, Bezmialem Vakif University Medical Faculty, Istanbul, Turkey. drfatihdilek@yahoo.com
- 2Department of Pediatric Allergy, Istanbul University Istanbul Medical Faculty, Istanbul, Turkey.
- 3Department of Clinical Biochemistry, Bezmialem Vakif University Medical Faculty, Istanbul, Turkey.
- 4Department of Pediatrics, Istanbul Bilim University, Istanbul, Turkey.
- KMID: 2349992
- DOI: http://doi.org/10.4168/aair.2016.8.6.522
Abstract
- PURPOSE
Chronic spontaneous urticaria (CSU) is a disease that is primarily seen in adults and is comparatively rare in children. Consequently, only a few studies have focused on the pathogenesis of the disease in children. This study investigated the possible role of metalloproteinase-9 (MMP-9) in the pathogenesis of CSU in children.
METHODS
The study group was composed of 54 children with CSU; 34 healthy children comprised the control group. The demographic and clinical features of the study group were extensively evaluated, and laboratory assessments were also performed. An enzyme-linked immunosorbent assay was used to evaluate levels of plasma MMP-9. Disease activity was quantified using the urticaria activity score (UAS).
RESULTS
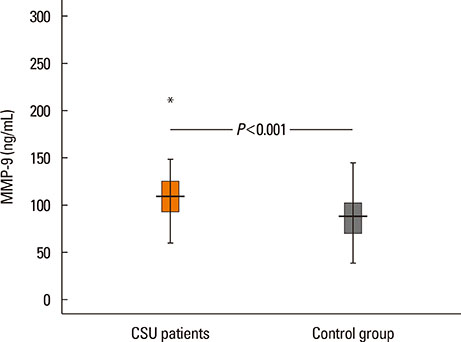
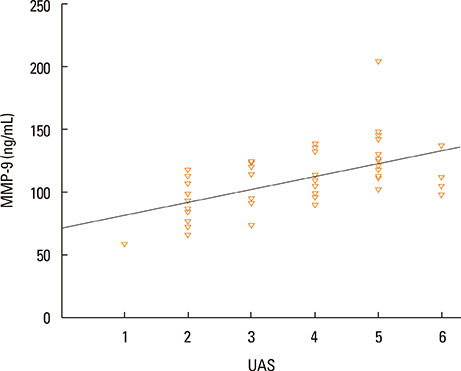
The median value of plasma MMP-9 was 108.9 ng/mL (interquartile range, 93.3-124.1) in the CSU group and 87.8 ng/mL (69.4-103.0) in the control group. The difference between the 2 groups was statistically significant (P<0.001). Also, MMP-9 levels showed a significant positive correlation with UAS (rho=0.57, P<0.001). Twenty-six percent of patients had positive autologous serum skin test (ASST) results. Neither UAS nor plasma MMP-9 levels were significantly different between ASST-positive and -negative patients (P>0.05).
CONCLUSIONS
Plasma MMP-9 levels were elevated in children with CSU and were positively correlated with disease activity. MMP-9 may be both a good biomarker of disease activity and a potential therapeutic target in CSU.
MeSH Terms
Figure
Reference
-
1. Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT. British Society for Allergy and Clinical Immunology. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015; 45:547–565.2. Maurer M, Weller K, Bindslev-Jensen C, Giménez-Arnau A, Bousquet PJ, Bousquet J, et al. Unmet clinical needs in chronic spontaneous urticaria. A GA2LEN task force report. Allergy. 2011; 66:317–330.3. Khakoo G, Sofianou-Katsoulis A, Perkin MR, Lack G. Clinical features and natural history of physical urticaria in children. Pediatr Allergy Immunol. 2008; 19:363–366.4. Volonakis M, Katsarou-Katsari A, Stratigos J. Etiologic factors in childhood chronic urticaria. Ann Allergy. 1992; 69:61–65.5. Ghosh S, Kanwar AJ, Kaur S. Urticaria in children. Pediatr Dermatol. 1993; 10:107–110.6. Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, et al. The EAACI/GA(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014; 69:868–887.7. Vestergaard C, Deleuran M. Chronic spontaneous urticaria: latest developments in aetiology, diagnosis and therapy. Ther Adv Chronic Dis. 2015; 6:304–313.8. Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015; 44-46:122–129.9. Ram M, Sherer Y, Shoenfeld Y. Matrix metalloproteinase-9 and autoimmune diseases. J Clin Immunol. 2006; 26:299–307.10. Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000; 96:2673–2681.11. Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β-induced peritonitis. J Immunol. 2013; 190:401–410.12. Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, et al. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2014; 306:H1398–H1407.13. Belleguic C, Corbel M, Germain N, Léna H, Boichot E, Delaval PH, et al. Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy. 2002; 32:217–223.14. Harper JI, Godwin H, Green A, Wilkes LE, Holden NJ, Moffatt M, et al. A study of matrix metalloproteinase expression and activity in atopic dermatitis using a novel skin wash sampling assay for functional biomarker analysis. Br J Dermatol. 2010; 162:397–403.15. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009; 64:1417–1426.16. Sabroe RA, Grattan CE, Francis DM, Barr RM, Kobza Black A, Greaves MW. The autologous serum skin test: a screening test for autoantibodies in chronic idiopathic urticaria. Br J Dermatol. 1999; 140:446–452.17. Church MK, Weller K, Stock P, Maurer M. Chronic spontaneous urticaria in children: itching for insight. Pediatr Allergy Immunol. 2011; 22:1–8.18. Jain S. Pathogenesis of chronic urticaria: an overview. Dermatol Res Pract. 2014; 2014:674709.19. Caproni M, Giomi B, Volpi W, Melani L, Schincaglia E, Macchia D, et al. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005; 114:284–292.20. Sun RS, Sui JF, Chen XH, Ran XZ, Yang ZF, Guan WD, et al. Detection of CD4+ CD25+ FOXP3+ regulatory T cells in peripheral blood of patients with chronic autoimmune urticaria. Australas J Dermatol. 2011; 52:e15–e18.21. Dos Santos JC, Azor MH, Nojima VY, Lourenço FD, Prearo E, Maruta CW, et al. Increased circulating pro-inflammatory cytokines and imbalanced regulatory T-cell cytokines production in chronic idiopathic urticaria. Int Immunopharmacol. 2008; 8:1433–1440.22. Kessel A, Bishara R, Amital A, Bamberger E, Sabo E, Grushko G, et al. Increased plasma levels of matrix metalloproteinase-9 are associated with the severity of chronic urticaria. Clin Exp Allergy. 2005; 35:221–225.23. Tedeschi A, Asero R, Lorini M, Marzano AV, Cugno M. Plasma levels of matrix metalloproteinase-9 in chronic urticaria patients correlate with disease severity and C-reactive protein but not with circulating histamine-releasing factors. Clin Exp Allergy. 2010; 40:875–881.24. Altrichter S, Boodstein N, Maurer M. Matrix metalloproteinase-9: a novel biomarker for monitoring disease activity in patients with chronic urticaria patients? Allergy. 2009; 64:652–656.25. Carr TF, Saltoun CA. Chapter 21: urticaria and angioedema. Allergy Asthma Proc. 2012; 33:Suppl 1. S70–S72.26. Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab. 2010; 30:837–848.27. Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015; 10:1040–1054.28. Herszényi L, Hritz I, Lakatos G, Varga MZ, Tulassay Z. The behavior of matrix metalloproteinases and their inhibitors in colorectal cancer. Int J Mol Sci. 2012; 13:13240–13263.29. Chaturvedi M, Kaczmarek L. Mmp-9 inhibition: a therapeutic strategy in ischemic stroke. Mol Neurobiol. 2014; 49:563–573.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Plasma Level of Matrix Metalloproteinase (MMP)-2, -9 between Antepartum and Postpartum Period in Preeclampsia
- The Effects of Hantaan Virus on Fibronectin and Matrix Metalloproteinase-3
- Expression of Matrix metalloproteinase-1 between Simple Chronic Periodontitis and Type 2 Diabetes associated Chronic Periodontitis on Protein level
- EGCC inhibits tumor growth by inbibiting Matrix Metalloproteinase-9 induction in UM-SCC-1 cells
- The effect of periodontal flap surgery on Matrix metalloproteinases (MMPs) and Tissue inhibitors of matrix metalloproteinase-1 (TIMP-1) levels in gingival crevicular fluids of periodontitis patients