Yonsei Med J.
2015 Nov;56(6):1478-1491. 10.3349/ymj.2015.56.6.1478.
Treatment of Retinoblastoma: The Role of External Beam Radiotherapy
- Affiliations
-
- 1Proton Therapy Center, National Cancer Center, Goyang, Korea. jooyoungcasa@ncc.re.kr
- KMID: 2345873
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1478
Abstract
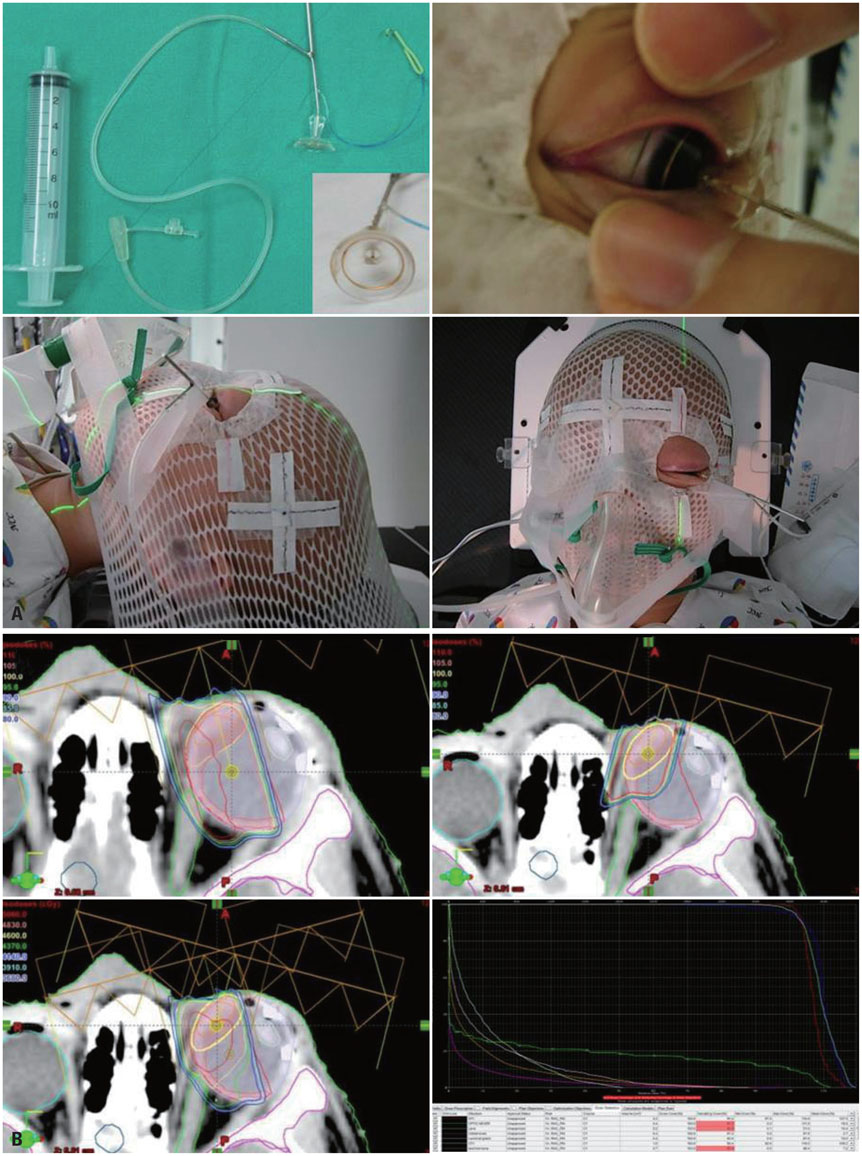
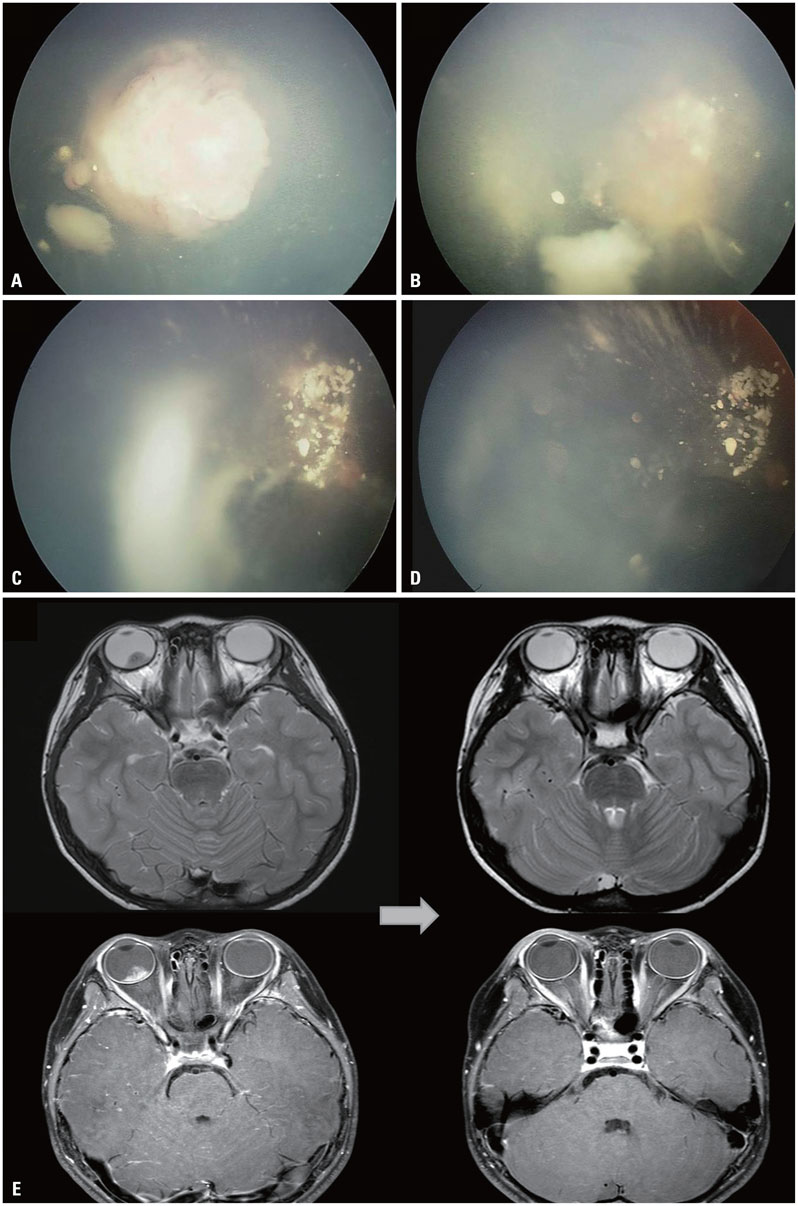
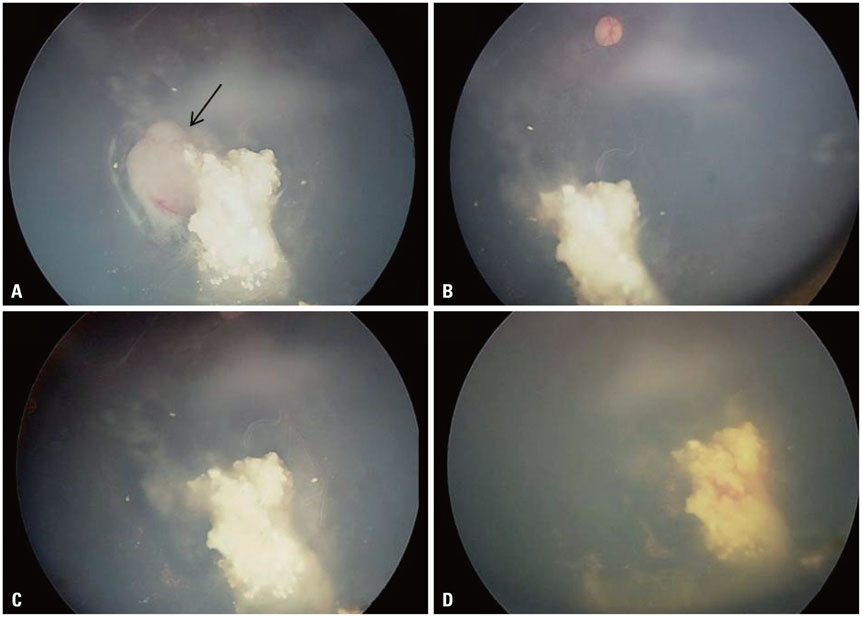
- The risk of radiotherapy-related secondary cancers in children with constitutional retinoblastoma 1 (RB1) mutations has led to reduced use of external beam radiotherapy (EBRT) for RB. Presently, tumor reduction with chemotherapy with or without focal surgery (chemosurgery) is most commonly undertaken; EBRT is avoided as much as possible and is considered only as the last treatment option prior to enucleation. Nevertheless, approximately 80% of patients are diagnosed at a locally advanced stage, and only 20-25% of early stage RB patients can be cured with a chemosurgery strategy. As a whole, chemotherapy fails in more than two-thirds of eyes with advanced stage disease, requiring EBRT or enucleation. Radiotherapy is still considered necessary for patients with large tumor(s) who are not candidates for chemosurgery but who have visual potential. When radiation therapy is indicated, the lowest possible radiation dose combined with systemic or local chemotherapy and focal surgery may yield the best clinical outcomes in terms of local control and treatment-related toxicity. Proton beam therapy is one EBRT method that can be used for treatment of RB and reduces the radiation dose delivered to the adjacent orbital bone while maintaining an adequate dose to the tumor. To maximize the therapeutic success of treatment of advanced RB, the possibility of integrating radiotherapy at early stages of treatment may need to be discussed by a multidisciplinary team, rather than considering EBRT as only a last treatment option.
MeSH Terms
Figure
Reference
-
1. Halperin EC, Constine LS, Tarbell NJ, Kun LE. Pediatric Radiation Oncology. Philadelphia: Wolters Kluwer Health;2012.2. Abramson DH. Retinoblastoma: saving life with vision. Annu Rev Med. 2014; 65:171–184.
Article3. Munier FL, Verwey J, Pica A, Balmer A, Zografos L, Abouzeid H, et al. New developments in external beam radiotherapy for retinoblastoma: from lens to normal tissue-sparing techniques. Clin Experiment Ophthalmol. 2008; 36:78–89.
Article4. Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, et al. Retinoblastoma. Lancet. 2012; 379:1436–1446.
Article5. Dimaras H, Dimba EA, Gallie BL. Challenging the global retinoblastoma survival disparity through a collaborative research effort. Br J Ophthalmol. 2010; 94:1415–1416.
Article6. Canadian Retinoblastoma Society. National Retinoblastoma Strategy Canadian Guidelines for Care: Stratégie thérapeutique du rétinoblastome guide clinique canadien. Can J Ophthalmol. 2009; 44:Suppl 2. S1–S88.7. Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology. 2014; 121:517–524.
Article8. Witkiewicz AK, Ertel A, McFalls J, Valsecchi ME, Schwartz G, Knudsen ES. RB-pathway disruption is associated with improved response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2012; 18:5110–5122.
Article9. Zhao J, Zhang Z, Liao Y, Du W. Mutation of the retinoblastoma tumor suppressor gene sensitizes cancers to mitotic inhibitor induced cell death. Am J Cancer Res. 2014; 4:42–52.10. Zhang M, Stevens G, Madigan MC. In vitro effects of radiation on human retinoblastoma cells. Int J Cancer. 2001; 96:Suppl. 7–14.
Article11. Svitra PP, Budenz D, Albert DM, Koehler AM, Gragoudas E. Proton beam irradiation for treatment of experimental human retinoblastoma. Eur J Ophthalmol. 1991; 1:57–62.
Article12. Cassady JR, Sagerman RH, Tretter P, Ellsworth RM. Radiation therapy in retinoblastoma. An analysis of 230 cases. Radiology. 1969; 93:405–409.13. Fontanesi J, Pratt CB, Hustu HO, Coffey D, Kun LE, Meyer D. Use of irradiation for therapy of retinoblastoma in children more than 1 year old: the St. Jude Children's Research Hospital experience and review of literature. Med Pediatr Oncol. 1995; 24:321–326.
Article14. Fontanesi J, Pratt C, Meyer D, Elverbig J, Parham D, Kaste S. Asynchronous bilateral retinoblastoma: the St. Jude Children's Research Hospital experience. Ophthalmic Genet. 1995; 16:109–112.
Article15. Berry JL, Jubran R, Kim JW, Wong K, Bababeygy SR, Almarzouki H, et al. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer. 2013; 60:688–693.
Article16. Shields CL, Ramasubramanian A, Thangappan A, Hartzell K, Leahey A, Meadows AT, et al. Chemoreduction for group E retinoblastoma: comparison of chemoreduction alone versus chemoreduction plus low-dose external radiotherapy in 76 eyes. Ophthalmology. 2009; 116:544–551.
Article17. Ramasubramanian A, Shields CL. Retinoblastoma. 1st ed. New Delhi: Jaypee Brothers Medical Publishers Ltd.;2012.18. Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Singh A, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol. 2002; 133:657–664.
Article19. Shields CL, Mashayekhi A, Cater J, Shelil A, Meadows AT, Shields JA. Chemoreduction for retinoblastoma. Analysis of tumor control and risks for recurrence in 457 tumors. Am J Ophthalmol. 2004; 138:329–337.
Article20. Shields CL, Fulco EM, Arias JD, Alarcon C, Pellegrini M, Rishi P, et al. Retinoblastoma frontiers with intravenous, intra-arterial, periocular, and intravitreal chemotherapy. Eye (Lond). 2013; 27:253–264.
Article21. Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol. 1996; 114:1339–1343.
Article22. Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007; 114:1378–1383.
Article23. Jairam V, Roberts KB, Yu JB. Historical trends in the use of radiation therapy for pediatric cancers: 1973-2008. Int J Radiat Oncol Biol Phys. 2013; 85:e151–e155.
Article24. Shinohara ET, DeWees T, Perkins SM. Subsequent malignancies and their effect on survival in patients with retinoblastoma. Pediatr Blood Cancer. 2014; 61:116–119.
Article25. Abramson DH, Beaverson KL, Chang ST, Dunkel IJ, McCormick B. Outcome following initial external beam radiotherapy in patients with Reese-Ellsworth group Vb retinoblastoma. Arch Ophthalmol. 2004; 122:1316–1323.
Article26. Blach LE, McCormick B, Abramson DH. External beam radiation therapy and retinoblastoma: long-term results in the comparison of two techniques. Int J Radiat Oncol Biol Phys. 1996; 35:45–51.
Article27. Foote RL, Garretson BR, Schomberg PJ, Buskirk SJ, Robertson DM, Earle JD. External beam irradiation for retinoblastoma: patterns of failure and dose-response analysis. Int J Radiat Oncol Biol Phys. 1989; 16:823–830.
Article28. Hernandez JC, Brady LW, Shields JA, Shields CL, DePotter P, Karlsson UL, et al. External beam radiation for retinoblastoma: results, patterns of failure, and a proposal for treatment guidelines. Int J Radiat Oncol Biol Phys. 1996; 35:125–132.
Article29. Desjardins L, Chefchaouni MC, Lumbroso L, Levy C, Asselain B, Bours D, et al. Functional results after treatment of retinoblastoma. J AAPOS. 2002; 6:108–111.
Article30. Messmer EP, Fritze H, Mohr C, Heinrich T, Sauerwein W, Havers W, et al. Long-term treatment effects in patients with bilateral retinoblastoma: ocular and mid-facial findings. Graefes Arch Clin Exp Ophthalmol. 1991; 229:309–314.
Article31. Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet. 2009; 374:1639–1651.
Article32. Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006; 113:2276–2280.
Article33. Künkele A, Jurklies C, Wieland R, Lohmann D, Bornfeld N, Eggert A, et al. Chemoreduction improves eye retention in patients with retinoblastoma: a report from the German Retinoblastoma Reference Centre. Br J Ophthalmol. 2013; 97:1277–1283.
Article34. Chan MP, Hungerford JL, Kingston JE, Plowman PN. Salvage external beam radiotherapy after failed primary chemotherapy for bilateral retinoblastoma: rate of eye and vision preservation. Br J Ophthalmol. 2009; 93:891–894.
Article35. Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol. 2009; 93:887–890.
Article36. Gündüz K, Shields CL, Shields JA, Meadows AT, Gross N, Cater J, et al. The outcome of chemoreduction treatment in patients with Reese-Ellsworth group V retinoblastoma. Arch Ophthalmol. 1998; 116:1613–1617.
Article37. Gündüz K, Günalp I, YalçindagXMLLink_XYZ N, Unal E, Taçyildiz N, Erden E, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004; 111:1917–1924.
Article38. Krengli M, Hug EB, Adams JA, Smith AR, Tarbell NJ, Munzenrider JE. Proton radiation therapy for retinoblastoma: comparison of various intraocular tumor locations and beam arrangements. Int J Radiat Oncol Biol Phys. 2005; 61:583–593.
Article39. Eldebawy E, Parker W, Abdel Rahman W, Freeman CR. Dosimetric study of current treatment options for radiotherapy in retinoblastoma. Int J Radiat Oncol Biol Phys. 2012; 82:e501–e505.
Article40. Sethi RV, Shih HA, Yeap BY, Mouw KW, Petersen R, Kim DY, et al. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014; 120:126–133.
Article41. Plowman PN, Kingston JE, Hungerford JL. Prophylactic retinal radiotherapy has an exceptional place in the management of familial retinoblastoma. Br J Cancer. 1993; 68:743–745.
Article42. Chantada G, Luna-Fineman S, Sitorus RS, Kruger M, Israels T, Leal-Leal C, et al. SIOP-PODC recommendations for graduated-intensity treatment of retinoblastoma in developing countries. Pediatr Blood Cancer. 2013; 60:719–727.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differences in radiotherapy application according to regional disease characteristics of hepatocellular carcinoma
- Long Term Follow-up Results of External Beam Radiotherapy as Primary Treatment for Retinoblastoma
- Three Cases of Trilateral Retinoblastoma
- Late-onset Osteosarcoma in Bilateral Retinoblastoma Survivor
- Korean Thyroid Association Guidelines on the Management of Differentiated Thyroid Cancers; Part I. Initial Management of Differentiated Thyroid Cancers - Chapter 7. Adjuvant External Beam Radiotherapy and Systemic Chemotherapy Following Thyroidectomy 2024