J Vet Sci.
2015 Sep;16(3):349-356. 10.4142/jvs.2015.16.3.349.
Air assisted lamellar keratectomy for the corneal haze model
- Affiliations
-
- 1Department of Veterinary Clinical Science, Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kmseo@snu.ac.kr
- 2Department of Veterinary Biomedical Science, Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 2344308
- DOI: http://doi.org/10.4142/jvs.2015.16.3.349
Abstract
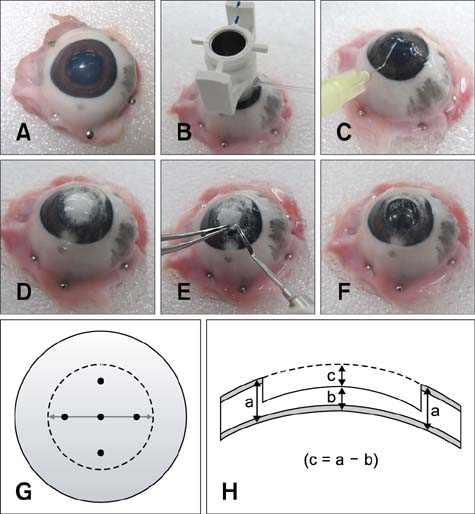
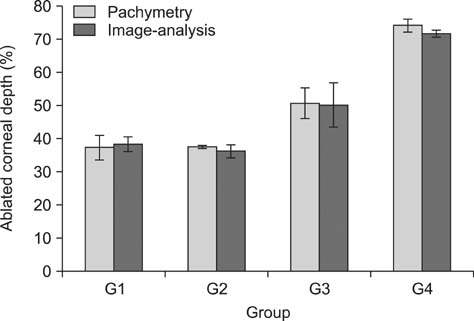
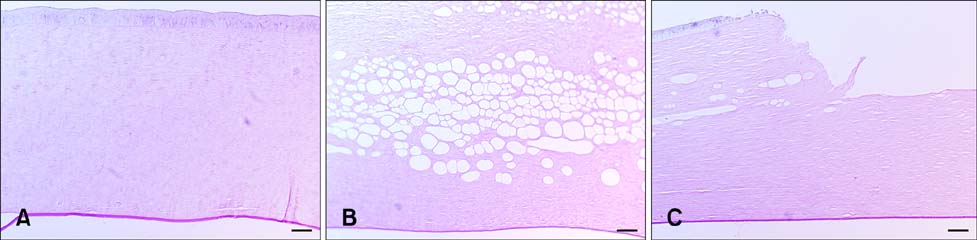
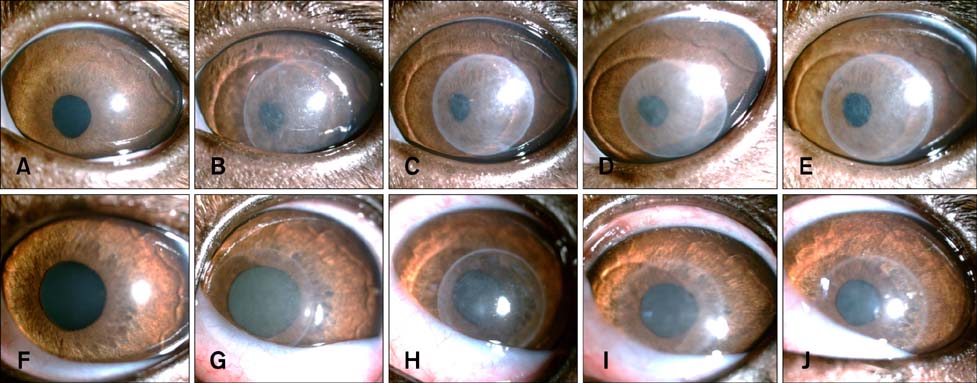
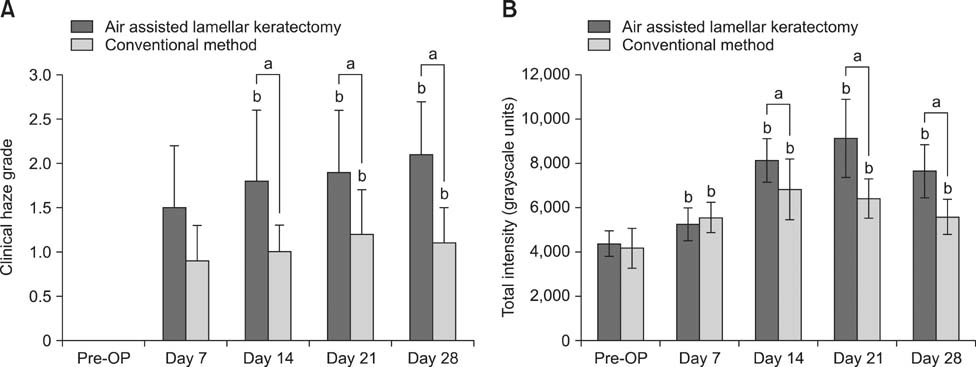
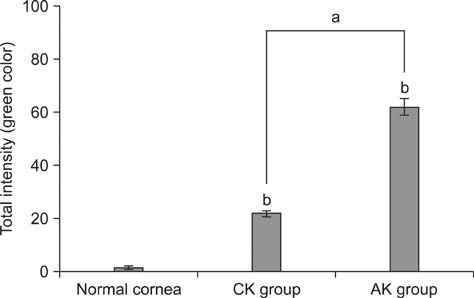
- To standardize the corneal haze model in the resection depth and size for efficient corneal haze development, air assisted lamellar keratectomy was performed. The ex vivo porcine corneas were categorized into four groups depending on the trephined depth: 250 microm (G1), 375 microm (G2), 500 microm (G3) and 750 microm (G4). The stroma was equally ablated at the five measurement sites in all groups. Significant differences were observed between the trephined corneal depths for resection and ablated corneal thickness in G1 (p < 0.001). No significant differences were observed between the trephined corneal depth for resection and the ablated corneal thickness in G2, G3, and G4. The resection percentage was similar in all groups after microscopic imaging of corneal sections. Air assisted lamellar keratectomy (AK) and conventional keratectomy (CK) method were applied to six beagles, after which development of corneal haze was evaluated weekly until postoperative day 28. The occurrence of corneal haze in the AK group was significantly higher than that in the CK group beginning 14 days after surgery. Alpha-smooth muscle actin expression was significantly higher in the AK group (p < 0.001) than the CK group. Air assisted lamellar keratectomy was used to achieve the desired corneal thickness after resection and produce sufficient corneal haze.
Keyword
MeSH Terms
Figure
Reference
-
1. Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002; 28:398–403.
Article2. Chang SW, Chou SF, Chuang JL. Mechanical corneal epithelium scraping and ethanol treatment up-regulate cytokine gene expression differently in rabbit cornea. J Refract Surg. 2008; 24:150–159.
Article3. de Medeiros FW, Mohan RR, Suto C, Sinhá S, Bonilha VL, Chaurasia SS, Wilson SE. Haze development after photorefractive keratectomy: mechanical vs ethanol epithelial removal in rabbits. J Refract Surg. 2008; 24:923–927.
Article4. Eckes B, Zweers MC, Zhang ZG, Hallinger R, Mauch C, Aumailley M, Krieg T. Mechanical tension and integrin α2β1 regulate fibroblast functions. J Investig Dermatol Symp Proc. 2006; 11:66–72.
Article5. Fantes FE, Hanna KD, Waring GO 3rd, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990; 108:665–675.
Article6. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999; 18:529–551.
Article7. Freund DE, McCally RL, Farrell RA, Cristol SM, L'Hernault NL, Edelhauser HF. Ultrastructure in anterior and posterior stroma of perfused human and rabbit corneas. Relation to transparency. Invest Ophthalmol Vis Sci. 1995; 36:1508–1523.8. Garrett Q, Khaw PT, Blalock TD, Scheltz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-β1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004; 45:1109–1116.
Article9. Hu C, Ding Y, Chen J, Liu D, Zhang Y, Ding M, Wang G. Basic fibroblast growth factor stimulates epithelial cell growth and epithelial wound healing in canine corneas. Vet Ophthalmol. 2009; 12:170–175.
Article10. Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of α-smooth muscle (α-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995; 36:809–819.11. McKee HD, Irion LCD, Carley FM, Jhanji V, Brahma AK. Dissection plane of the clear margin big-bubble in deep anterior lamellar keratoplasty. Cornea. 2013; 32:e51–e52.
Article12. Lin N, Yee SB, Mitra S, Chuang AZ, Yee RW. Prediction of corneal haze using an ablation depth/corneal thickness ratio after laser epithelial keratomileusis. J Refract Surg. 2004; 20:797–802.
Article13. Meeka KM, Fullwood NJ. Corneal and scleral collagens: a microscopist's perspective. Micron. 2001; 32:261–272.14. Mohan RR, Hutcheon AEK, Choi R, Hong J, Lee J, Mohan RR, Ambrósio R Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003; 76:71–87.
Article15. Mohan RR, Stapleton WM, Sinha S, Netto MV, Wilson SE. A novel method for generating corneal haze in anterior stroma of the mouse eye with the excimer laser. Exp Eye Res. 2008; 86:235–240.
Article16. Møller-Pedersen T. Keratocyte reflectivity and corneal haze. Exp Eye Res. 2004; 78:553–560.
Article17. Rieck P, Assouline M, Savoldelli M, Hartmann C, Jacob C, Pouliquen Y, Courtois Y. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: corneal wound healing effect and pharmacology. Exp Eye Res. 1992; 54:987–998.
Article18. Safianik B, Ben-Zion I, Garzozi HJ. Serious corneoscleral complications after pterygium excision with mitomycin C. Br J Ophthalmol. 2002; 86:357–358.
Article19. Sakimoto T, Rosenblatt MI, Azar DT. Laser eye surgery for refractive errors. Lancet. 2006; 367:1432–1447.
Article20. Soong HK, Malta JB, Mian SI, Juhasz T. Femtosecond laser-assisted lamellar keratopalsty. Arq Bras Oftalmol. 2008; 71:601–606.21. Stepp MA, Zieske JD, Trinkaus-Randall V, Kyne BM, Pal-Ghosh S, Tadvalkar G, Pajoohesh-Ganji A. Wounding the cornea to learn how it heals. Exp Eye Res. 2014; 121:178–193.
Article22. Teus MA, de Benito-Llopis L, Alió JL. Mitomycin C in corneal refractive surgery. Surv Ophthalmol. 2009; 54:487–502.
Article23. Yang G, Espandar L, Mamalis N, Prestwich GD. A cross-linked hyaluronan gel accelerates healing of corneal epithelial abrasion and alkali burn injuries in rabbits. Vet Ophthalmol. 2010; 13:144–150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corneal Haze after Excimer Laser Photorefractive Keratectomy for Myopia
- Effects of Topical Tranilast on Corneal Haze with the Pentacam(R) after Photorefractive Keratectomy
- Comparison of Photorefractive Keratectomy and Laser Epithelial Keratomileusis for Low to Moderate Myopia
- Case Report: Femtosecond Laser-Assisted Small Incision Deep Lamellar Endothelial Keratoplasty
- The Use of Ascorbic Acid after Excimer Laser Photo refractive Keratectomy in Rabbits