J Korean Med Sci.
2015 Oct;30(10):1522-1530. 10.3346/jkms.2015.30.10.1522.
Dosimetric Effects of Magnetic Resonance Imaging-assisted Radiotherapy Planning: Dose Optimization for Target Volumes at High Risk and Analytic Radiobiological Dose Evaluation
- Affiliations
-
- 1Department of Radiation Oncology, University of Florida, FL, USA.
- 2Department of Biomedical Engineering, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Research Institute of Biomedical Engineering, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Department of Radiation Oncology, Konkuk University Medical Center, Seoul, Korea. semiehong@kuh.ac.kr
- 5Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2344189
- DOI: http://doi.org/10.3346/jkms.2015.30.10.1522
Abstract
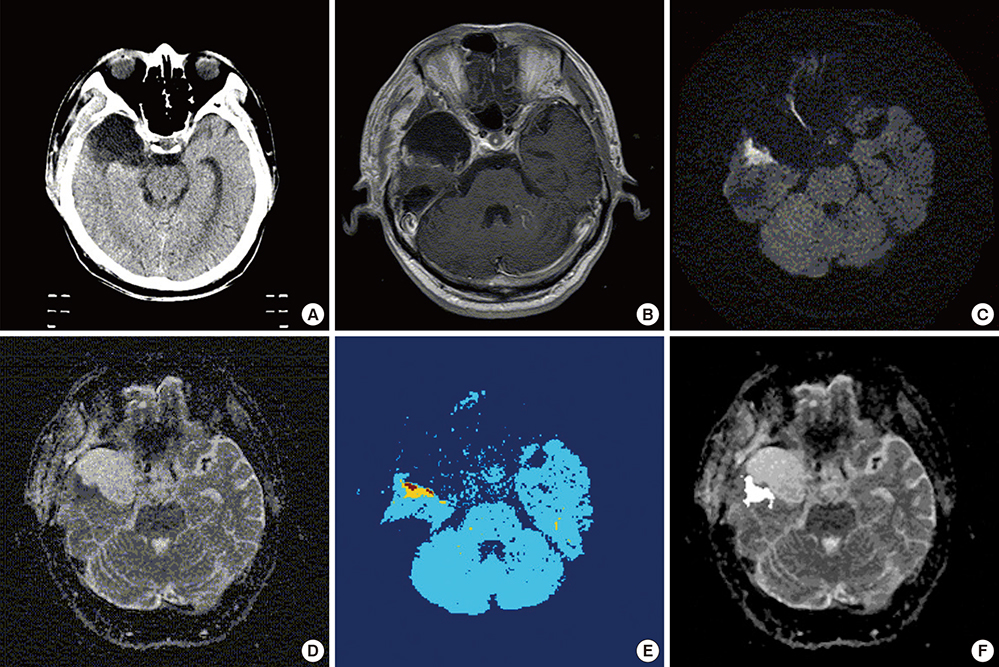
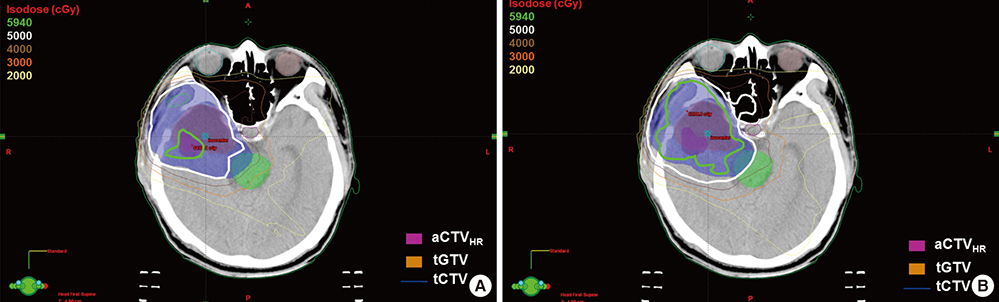
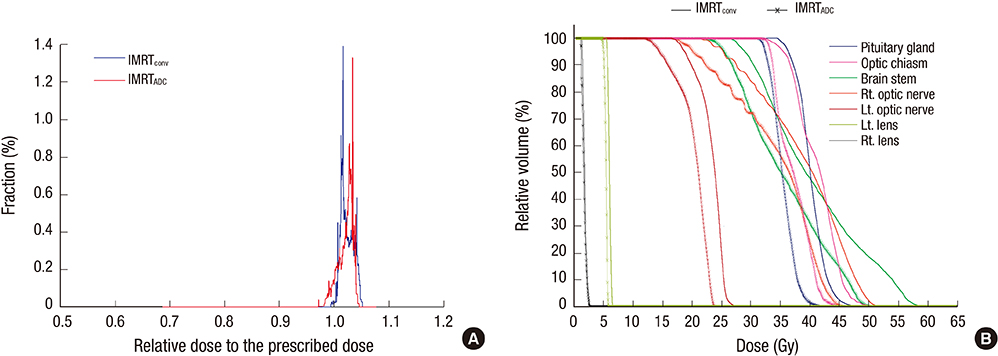
- Based on the assumption that apparent diffusion coefficients (ADCs) define high-risk clinical target volume (aCTVHR) in high-grade glioma in a cellularity-dependent manner, the dosimetric effects of aCTVHR-targeted dose optimization were evaluated in two intensity-modulated radiation therapy (IMRT) plans. Diffusion-weighted magnetic resonance (MR) images and ADC maps were analyzed qualitatively and quantitatively to determine aCTVHR in a high-grade glioma with high cellularity. After confirming tumor malignancy using the average and minimum ADCs and ADC ratios, the aCTVHR with double- or triple-restricted water diffusion was defined on computed tomography images through image registration. Doses to the aCTVHR and CTV defined on T1-weighted MR images were optimized using a simultaneous integrated boost technique. The dosimetric benefits for CTVs and organs at risk (OARs) were compared using dose volume histograms and various biophysical indices in an ADC map-based IMRT (IMRTADC) plan and a conventional IMRT (IMRTconv) plan. The IMRTADC plan improved dose conformity up to 15 times, compared to the IMRTconv plan. It reduced the equivalent uniform doses in the visual system and brain stem by more than 10% and 16%, respectively. The ADC-based target differentiation and dose optimization may facilitate conformal dose distribution to the aCTVHR and OAR sparing in an IMRT plan.
Keyword
MeSH Terms
Figure
Reference
-
1. Stieber VW, Munley MT. Central nervous system tumors overview. In : Mundt AJ, Roeske JC, editors. Intensity modulated radiation therapy: a clinical perspective. New York: BC Decker;2005. p. 231–240.2. Dai CY, Nakamura JL, Haas-Kogan D, Larson DA. Central nervous system. In : Hansen EK, Roach M, editors. Handbook of evidence-based radiation oncology. New York: Springer;2007. p. 15–54.3. Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology. 2002; 59:947–949.4. Fan GG, Deng QL, Wu ZH, Guo QY. Usefulness of diffusion/perfusion-weighted MRI in patients with non-enhancing supratentorial brain gliomas: a valuable tool to predict tumour grading? Br J Radiol. 2006; 79:652–658.5. Xing L, Cotrutz C, Hunjan S, Boyer AL, Adalsteinsson E, Spielman D. Inverse planning for functional image-guided intensity-modulated radiation therapy. Phys Med Biol. 2002; 47:3567–3578.6. Chang J, Thakur S, Perera G, Kowalski A, Huang W, Karimi S, Hunt M, Koutcher J, Fuks Z, Amols H, et al. Image-fusion of MR spectroscopic images for treatment planning of gliomas. Med Phys. 2006; 33:32–40.7. Narayana A, Chang J, Thakur S, Huang W, Karimi S, Hou B, Kowalski A, Perera G, Holodny A, Gutin PH. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br J Radiol. 2007; 80:347–354.8. Fuller CD, Choi M, Forthuber B, Wang SJ, Rajagiriyil N, Salter BJ, Fuss M. Standard fractionation intensity modulated radiation therapy (IMRT) of primary and recurrent glioblastoma multiforme. Radiat Oncol. 2007; 2:26.9. Suzuki M, Nakamatsu K, Kanamori S, Okumra M, Uchiyama T, Akai F, Nishimura Y. Feasibility study of the simultaneous integrated boost (SIB) method for malignant gliomas using intensity-modulated radiotherapy (IMRT). Jpn J Clin Oncol. 2003; 33:271–277.10. Chan JL, Lee SW, Fraass BA, Normolle DP, Greenberg HS, Junck LR, Gebarski SS, Sandler HM. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002; 20:1635–1642.11. Hamstra DA, Rehemtulla A, Ross BD. Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol. 2007; 25:4104–4109.12. Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008; 359:492–507.13. Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999; 9:53–60.14. Catalaa I, Henry R, Dillon WP, Graves EE, McKnight TR, Lu Y, Vigneron DB, Nelson SJ. Perfusion, diffusion and spectroscopy values in newly diagnosed cerebral gliomas. NMR Biomed. 2006; 19:463–475.15. Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr. 2004; 28:735–746.16. Stieber VW, McMullen KP, DeGuzman A, Shaw EG. Cancer of the central nervous system. In : Khan FM, editor. Treatment planning in radiation oncology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;2006. p. 410–428.17. Brem SS, Bierman PJ, Brem H, Butowski N, Chamberlain MC, Chiocca EA, DeAngelis LM, Fenstermaker RA, Friedman A, Gilbert MR, et al. Central nervous system cancers. J Natl Compr Canc Netw. 2011; 9:352–400.18. Young GS, Xia S. Advanced MR techniques in clinical brain tumor imaging. In : Newton HB, Jolesz FA, Malkin MG, Bourekas E, Christoforidis G, editors. Handbook of neuro-oncology neuroimaging. New York: Academic Press;2008. p. 136–149.19. In : Barrett A, Dobbs J, Morris S, Roques T, editors. Chapter18, Central nervous system. Practical radiotherapy planning. 4th ed. London: Hodder Arnold;2009. p. 205–230.20. Yoon M, Park SY, Shin D, Lee SB, Pyo HR, Kim DY, Cho KH. A new homogeneity index based on statistical analysis of the dose-volume histogram. J Appl Clin Med Phys. 2007; 8:9–17.21. Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys. 2006; 64:333–342.22. Ebert MA. Viability of the EUD and TCP concepts as reliable dose indicators. Phys Med Biol. 2000; 45:441–457.23. Hobbs RF, Baechler S, Fu DX, Esaias C, Pomper MG, Ambinder RF, Sgouros G. A model of cellular dosimetry for macroscopic tumors in radiopharmaceutical therapy. Med Phys. 2011; 38:2892–2903.24. Qi XS, Semenenko VA, Li XA. Improved critical structure sparing with biologically based IMRT optimization. Med Phys. 2009; 36:1790–1799.25. Warkentin B, Stavrev P, Stavreva N, Field C, Fallone BG. A TCP-NTCP estimation module using DVHs and known radiobiological models and parameter sets. J Appl Clin Med Phys. 2004; 5:50–63.26. Gay HA, Niemierko A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys Med. 2007; 23:115–125.27. Suit HD, Baumann M, Skates S, Convery K. Clinical interest in determinations of cellular radiation sensitivity. Int J Radiat Biol. 1989; 56:725–737.28. MacDonald SM, Ahmad S, Kachris S, Vogds BJ, DeRouen M, Gittleman AE, DeWyngaert K, Vlachaki MT. Intensity modulated radiation therapy versus three-dimensional conformal radiation therapy for the treatment of high grade glioma: a dosimetric comparison. J Appl Clin Med Phys. 2007; 8:47–60.29. Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Wakasa K, Yamada R. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001; 22:1081–1088.30. Moffat BA, Chenevert TL, Lawrence TS, Meyer CR, Johnson TD, Dong Q, Tsien C, Mukherji S, Quint DJ, Gebarski SS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A. 2005; 102:5524–5529.31. Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008; 18:1937–1952.32. Metcalfe P, Liney GP, Holloway L, Walker A, Barton M, Delaney GP, Vinod S, Tome W. The potential for an enhanced role for MRI in radiation-therapy treatment planning. Technol Cancer Res Treat. 2013; 12:429–446.33. Tsien C, Moughan J, Michalski JM, Gilbert MR, Purdy J, Simpson J, Kresel JJ, Curran WJ, Diaz A, Mehta MP. Phase I three-dimensional conformal radiation dose escalation study in newly diagnosed glioblastoma: Radiation Therapy Oncology Group Trial 98-03. Int J Radiat Oncol Biol Phys. 2009; 73:699–708.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Techniques for Optimal Treatment Planning for LINAC-based Sterotactic Radiosurgery
- Dosimetric and Radiobiological Evaluation of Dose Volume Optimizer (DVO) and Progressive Resolution Optimizer (PRO) Algorithm against Photon Optimizer on IMRT and VMAT Plan for Prostate Cancer
- Clinical outcome after high dose rate intracavitary brachytherapy with traditional point ‘A’ dose prescription in locally advanced carcinoma of uterine cervix: dosimetric analysis from the perspective of computed tomography imaging-based 3-dimensional treatment planning
- Dosimetric Effects of Air Pocket during Magnetic Resonance-Guided Adaptive Radiation Therapy for Pancreatic Cancer
- Dosimetric evaluation of magnetic resonance imaging-guided adaptive radiation therapy in pancreatic cancer by extent of re-contouring of organs-at-risk