Cancer Res Treat.
2016 Jul;48(3):1056-1064. 10.4143/crt.2015.282.
Serum Concentrations of Selenium and Copper in Patients Diagnosed with Pancreatic Cancer
- Affiliations
-
- 1Department of Genetics and Pathology, International Hereditary Cancer Center, Pomeranian Medical University, Szczecin, Poland. marcinlener@poczta.onet.pl
- 2Discipline of Medical Genetics, School of Biomedical Sciences, Faculty of Health, University of Newcastle and The Hunter Medical Research Institute, Newcastle, New South Wales, Australia.
- 3Laboratory of Endoscopy, Division of Heath Care Ministry of Internal Affairs and Administration, Szczecin, Poland.
- 4Read-Gene S.A., Grzepnica, Pomeranian Medical University, Szczecin, Poland.
- 5Department of General and Oncological Surgery, Pomeranian Medical University, Szczecin, Poland.
- KMID: 2344079
- DOI: http://doi.org/10.4143/crt.2015.282
Abstract
- PURPOSE
Understanding of the etiology and pathogenesis of pancreatic cancer (PaCa) is still insufficient. This study evaluated the associations between concentrations of selenium (Se) and copper (Cu) in the serum of PaCa patients.
MATERIALS AND METHODS
The study included 100 PaCa patients and 100 control subjects from the same geographical region in Poland. To determine the average concentration of Se, Cu, and ratio Cu:Se in the Polish population, assay for Se and Cu was performed in 480 healthy individuals. Serum levels of Se and Cu were measured using inductively coupled plasma mass spectrometry.
RESULTS
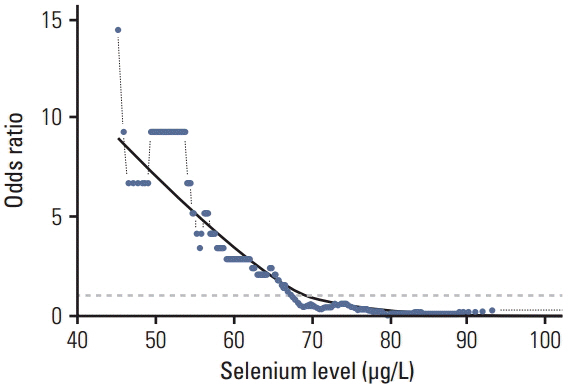
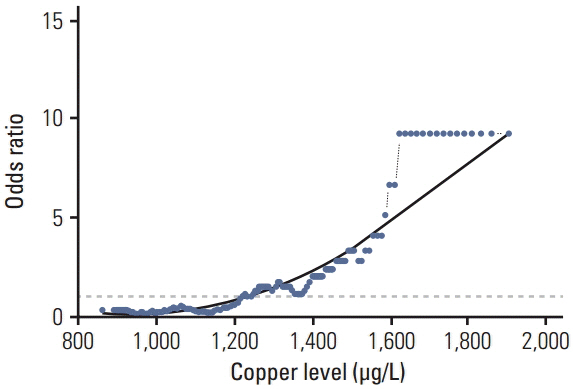
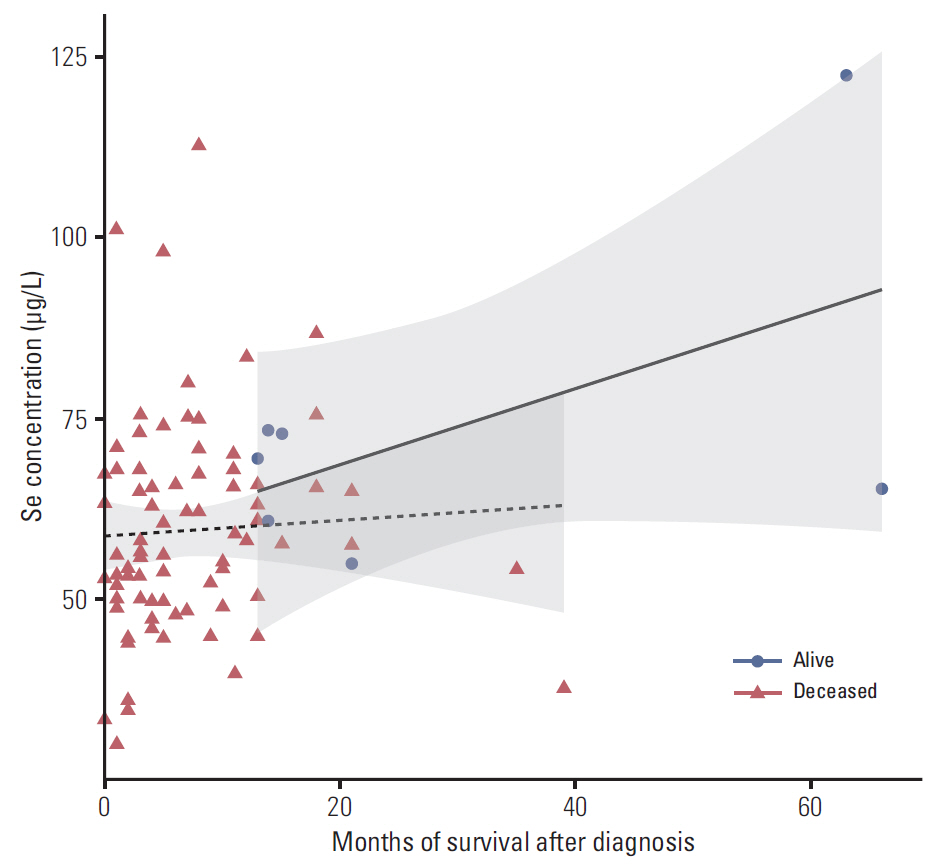
In the control group, the average Se level was 76 µg/L and Cu 1,098 µg/L. The average Se level among PaCa patients was 60 µg/L and the mean Cu level was 1,432 µg/L. The threshold point at which any decrease in Se concentration was associated with PaCa was 67.45 µg/L. The threshold point of Cu level above which there was an increase in the prevalence of PaCa was 1,214.58 µg/L. In addition, a positive relationship was observed between increasing survival time and Se plasma level.
CONCLUSION
This retrospective study suggests that low levels of Se and high levels of Cu might influence development of PaCa and that higher levels of Se are associated with longer survival in patients with PaCa. The results suggest that determining the level of Se and Cu could be incorporated into a risk stratification scheme for the selection and surveillance control examination to complement existing screening and diagnostic procedures.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015; 518:495–501.2. Silverman DT, Dunn JA, Hoover RN, Schiffman M, Lillemoe KD, Schoenberg JB, et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1994; 86:1510–6.
Article3. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004; 363:1049–57.
Article4. Goggins M, Schutte M, Lu J, Moskaluk CA, Weinstein CL, Petersen GM, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res. 1996; 56:5360–4.5. Klein AP, Lindstrom S, Mendelsohn JB, Steplowski E, Arslan AA, Bueno-de-Mesquita HB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One. 2013; 8:e72311.
Article6. Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009; 41:986–90.7. Petersen GM, Amundadottir L, Fuchs CS, Kraf P, Stolzenberg-Solomon RZ, Jacobs KB, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010; 42:224–8.8. Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004; 64:2634–8.
Article9. Chan S, Gerson B, Subramaniam S. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin Lab Med. 1998; 18:673–85.
Article10. Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs Gf Jr, Slate EH, Fischbach LA, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002; 11:630–9.11. Dennert G, Zwahlen M, Brinkman M, Vinceti M, Zeegers MP, Horneber M. Selenium for preventing cancer. Cochrane Database Syst Rev. 2011; (5):CD005195.
Article12. Rayman MP. Selenium and human health. Lancet. 2012; 379:1256–68.
Article13. Rayman MP, Stranges S. Epidemiology of selenium and type 2 diabetes: can we make sense of it? Free Radic Biol Med. 2013; 65:1557–64.
Article14. Linder MC, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996; 63:797S–811S.15. Kodydkova J, Vavrova L, Stankova B, Macasek J, Krechler T, Zak A. Antioxidant status and oxidative stress markers in pancreatic cancer and chronic pancreatitis. Pancreas. 2013; (42):614–21.
Article16. Evans DM, Zhu G, Dy V, Heath AC, Madden PA, Kemp JP, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet. 2013; (22):3998–4006.
Article17. Leung PS, Chan YC. Role of oxidative stress in pancreatic inflammation. Antioxid Redox Signal. 2009; 11:135–65.
Article18. Skipworth JR, Szabadkai G, Olde Damink SW, Leung PS, Humphries SE, Montgomery HE. Review article: pancreatic renin-angiotensin systems in health and disease. Aliment Pharmacol Ther. 2011; 34:840–52.
Article19. Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal. 2011; 15:2757–66.
Article20. Amaral AF, Porta M, Silverman DT, Milne RL, Kogevinas M, Rothman N, et al. Pancreatic cancer risk and levels of trace elements. Gut. 2012; 61:1583–8.
Article21. Burney PG, Comstock GW, Morris JS. Serologic precursors of cancer: serum micronutrients and the subsequent risk of pancreatic cancer. Am J Clin Nutr. 1989; 49:895–900.
Article22. Farzin L, Moassesi ME, Sajadi F, Faghih MA. Evaluation of trace elements in pancreatic cancer patients in Iran. Middle East J Cancer. 2013; 4:79–86.23. Bandyopadhyay U, Das D, Banerjee RK. Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci. 1999; 77:658–66.24. Manousos O, Trichopoulos D, Koutselinis A, Papadimitriou C, Polychronopoulou A, Zavitsanos X. Epidemiologic characteristics and trace elements in pancreatic cancer in Greece. Cancer Detect Prev. 1981; 4:439–42.25. Banim PJ, Luben R, McTaggart A, Welch A, Wareham N, Khaw KT, et al. Dietary antioxidants and the aetiology of pancreatic cancer: a cohort study using data from food diaries and biomarkers. Gut. 2013; 62:1489–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Concentration of Antioxidant Minerals in Cataract Patients
- The Serum Selenium Level in Korean Men and Its Association with Age and Prostate Cancer
- Longitudinal Study on Trace Mineral Compositions (Selenium, Zinc, Copper, Manganese) in Korean Human Preterm Milk
- Micronutrient Deficiency Syndrome: Zinc, Copper and Selenium
- Clinical Correlation between Gastric Cancer Type and Serum Selenium and Zinc Levels