J Korean Ophthalmol Soc.
2015 Jun;56(6):906-910. 10.3341/jkos.2015.56.6.906.
Adherence to Preservative-Free Dorzolamide/Timolol Fixed Combination Assessed by Counting the Unused Single-Dose Units
- Affiliations
-
- 1Department of Ophthalmology, Korea University College of Medicine, Seoul, Korea. augen@lycos.co.kr
- KMID: 2339156
- DOI: http://doi.org/10.3341/jkos.2015.56.6.906
Abstract
- PURPOSE
To investigate the actual adherence to treatment with preservative-free dorzolamide-timolol fixed combination (DTFC) eyedrops of primary open-angle glaucoma (POAG) patients by counting the number of unused single-dose units of DTFC.
METHODS
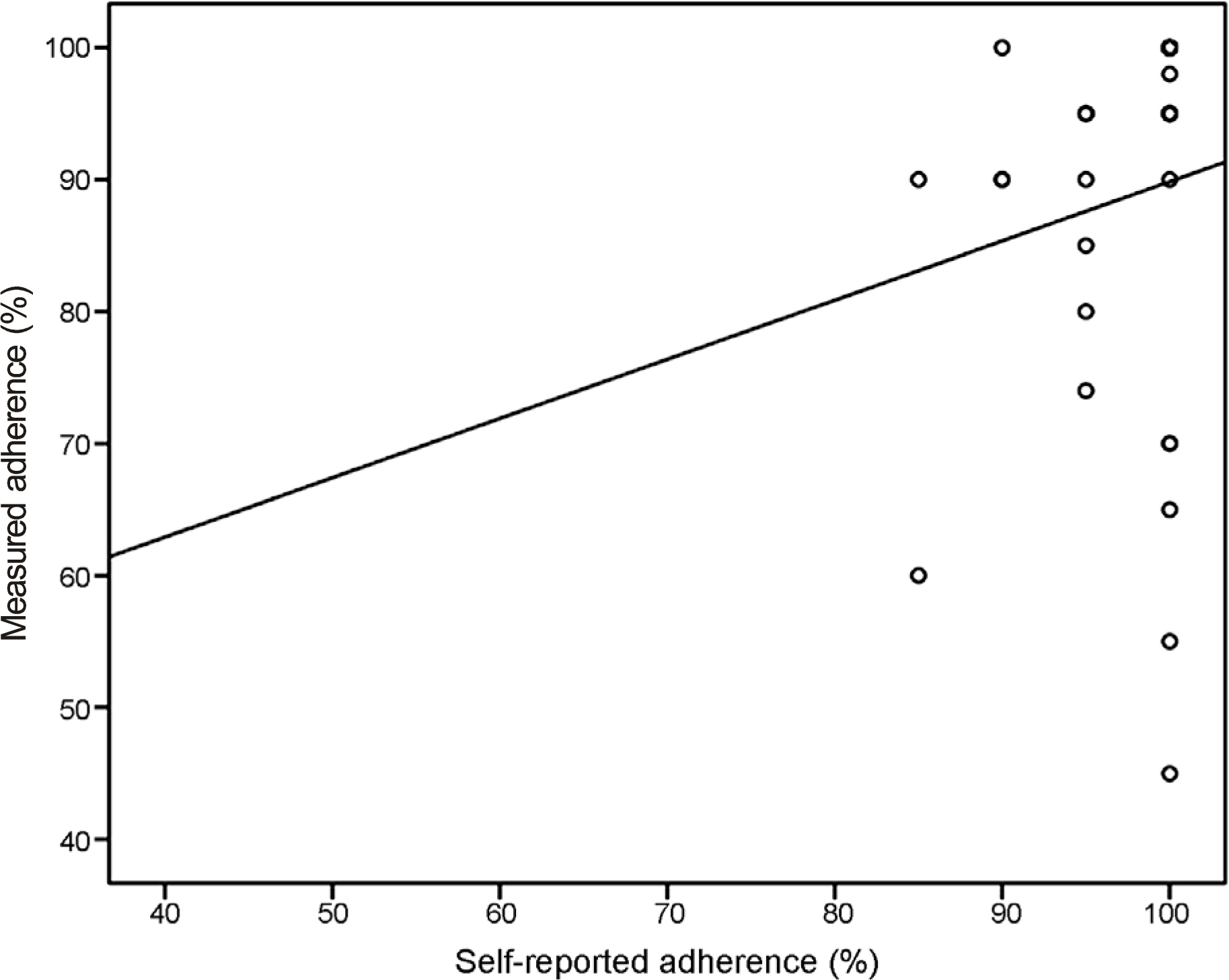
This study included 34 POAG patients newly prescribed with preservative-free DTFC eyedrops (formulated in single-dose units). The enrolled patients were asked to bring the unused DTFC units on their next visit after 2 weeks of treatment with DTFC. On their second visit, they were asked to complete a questionnaire regarding the self-reported adherence and the number of unused DTFC single-dose units was counted. The actual adherence (%) was calculated by dividing the expected number of used DTFC units by the actual number of used DTFC units. The correlation between the self-reported adherence and the measured adherence was assessed.
RESULTS
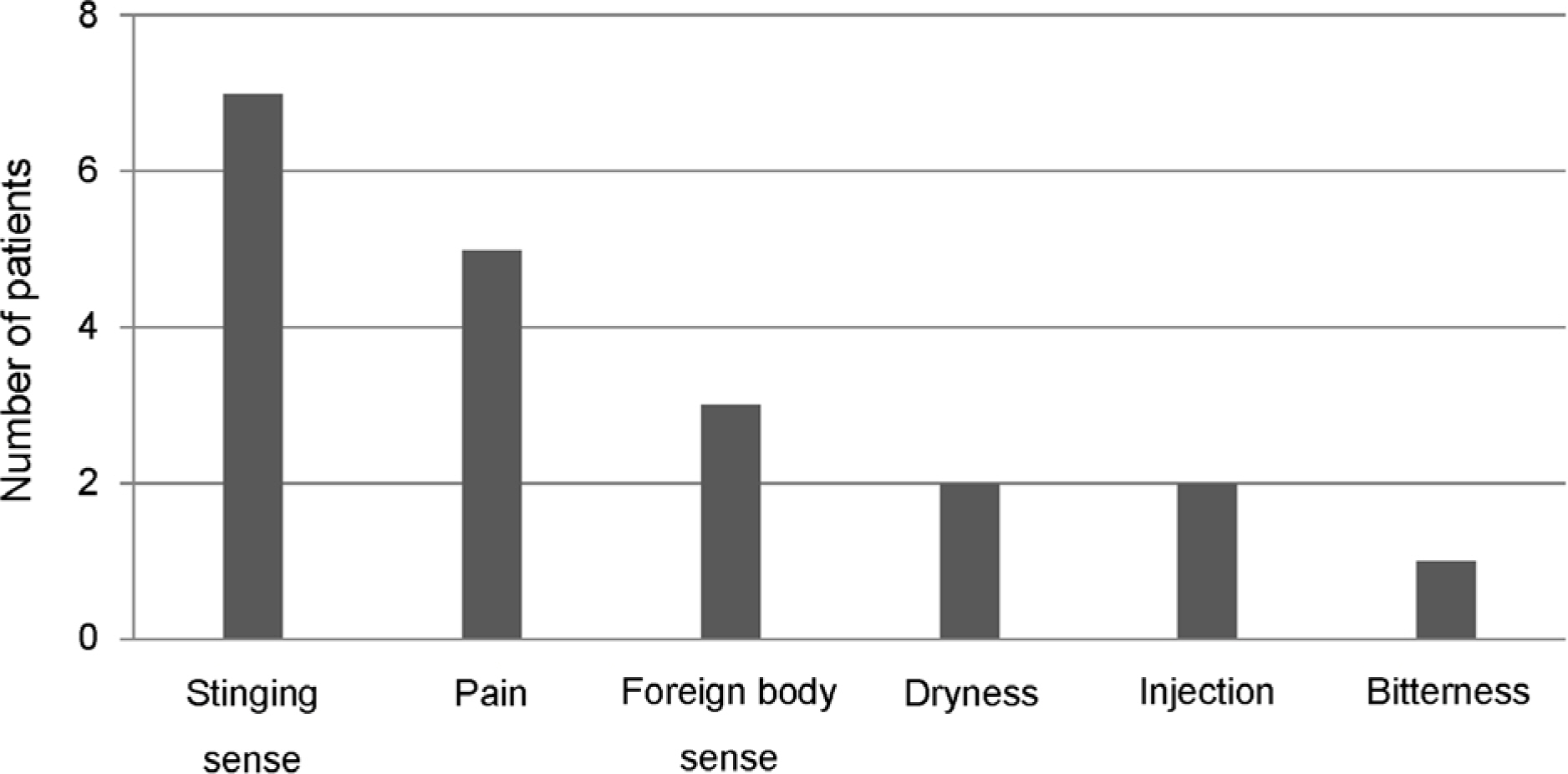
Twenty-nine (93.5%) patients answered they adhered to the medication by more than 90% and 2 (6.5%) answered they instilled the eyedrops at 80-90% of the dosing schedule. However, after counting the unused DTFC single-dose units, 9 (29.0%) patients showed an actual adherence of <90%. Moreover, the actual adherence of 3 (9.7%) patients was <60%. Unexpectedly, 4 (12.9%) patients showed the actual adherence exceeding 100% (196%, 1 patient; 107-132%, 3 patients).
CONCLUSIONS
We demonstrated a large difference between the self-reported and the actual adherence to treatment by counting the unused single-dose units of eyedrops. Preservative-free topical anti-glaucoma medications (formulated in single-dose units) provide clinicians an opportunity to assess the actual adherence of glaucoma patients by counting the unused units of eyedrops.
MeSH Terms
Figure
Cited by 2 articles
-
Effect of a Preservative-free Dorzolamide/Timolol Fixed Combination on Elevated Intraocular Pressure after Vitrectomy
Sung Hoon Lee, Wonseok Lee, Gong Je Seong, Suk Ho Byeon, Sung Soo Kim, Hyoung Jun Koh, Sung Chul Lee, Min Kim
J Korean Ophthalmol Soc. 2016;57(9):1386-1391. doi: 10.3341/jkos.2016.57.9.1386.Comparison of Allergy Prevalence between Brimonidine/Timolol Fixed Combination and 0.15% Brimonidine in Glaucoma Patients
Eun Jung Park, Yeoun Sook Chun
J Korean Ophthalmol Soc. 2018;59(5):451-458. doi: 10.3341/jkos.2018.59.5.451.
Reference
-
References
1. Kingman S. Glaucoma is second leading cause of blindness glo-bally. Bull World Health Organ. 2004; 82:887–8.2. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–13. discussion 829-30.3. Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998; 126:498–505.4. Friedman DS, Wilson MR, Liebmann JM, et al. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004; 138(3 Suppl):S19–31.
Article5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353:487–97.
Article6. Ashburn FS Jr, Goldberg I, Kass MA. Compliance with ocular therapy. Surv Ophthalmol. 1980; 24:237–48.
Article7. DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004; 42:200–9.8. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008; 53(Suppl 1):S57–68.
Article9. Kist K. Basis of quantitative perimetry. Anderson DR, Patella VM, editors. Automated static perimetry. 2nd ed.St. Louis: Mosby;1998. chap. 2.10. Feuer WJ. Glaucomatous visual field loss. Hodapp E, Parrish RK, Anderson DR, editors. Clinical decisions in glaucoma. 1st ed.St. Louis: Mosby;1993. chap. 2.11. Beckers HJ, Schouten JS, Webers CA, et al. Side effects of com-monly used glaucoma medications: comparison of tolerability, chance of discontinuation, and patient satisfaction. Graefes Arch Clin Exp Ophthalmol. 2008; 246:1485–90.
Article12. Hahn SR. Patient-centered communication to assess and enhance patient adherence to glaucoma medication. Ophthalmology. 2009; 116(11 Suppl):S37–42.
Article13. Ahn DH, Lee YG, Hong YJ. Factors affecting compliance with prescribed eyedrops for glaucoma. J Korean Ophthalmol Soc. 1998; 39:2145–51.14. Park MH, Kang KD, Moon J; Korean Glaucoma Compliance Study Group. Noncompliance with glaucoma medication in Korean patients: a multicenter qualitative study. Jpn J Ophthalmol. 2013; 57:47–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy and Safety of Preservative-containing and Preservative-free Brimonidine-Timolol Fixed Combination in Normal Tension Glaucoma
- Comparison of Cytotoxic Effects on Rabbit Corneal Endothelium between Preservative-free and Preservative-containing Dorzolamide/timolol
- The Effect of Antiglaucoma Medication on Cultured Human Conjunctival Epithelial Cells
- Effect of a Preservative-free Dorzolamide/Timolol Fixed Combination on Elevated Intraocular Pressure after Vitrectomy
- Add-on Effect of Prostaglandin Analogues in Dorzolamide/Timolol Fixed Combination Treated Primary Open Angle Glaucoma Patients