J Periodontal Implant Sci.
2013 Dec;43(6):251-261.
Advances in the design of macroporous polymer scaffolds for potential applications in dentistry
- Affiliations
-
- 1School of Engineering and Applied Sciences, Harvard University, Cambridge, MA, USA. sidi@seas.harvard.edu
- 2Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston, MA, USA.
- 3Laboratory of Microsystems, STI-LMIS4, Ecole Polytechnique Federale de Lausanne (EPFL), Lausanne, Switzerland.
Abstract
- A paradigm shift is taking place in medicine and dentistry from using synthetic implants and tissue grafts to a tissue engineering approach that uses degradable porous three-dimensional (3D) material hydrogels integrated with cells and bioactive factors to regenerate tissues such as dental bone and other oral tissues. Hydrogels have been established as a biomaterial of choice for many years, as they offer diverse properties that make them ideal in regenerative medicine, including dental applications. Being highly biocompatible and similar to native extracellular matrix, hydrogels have emerged as ideal candidates in the design of 3D scaffolds for tissue regeneration and drug delivery applications. However, precise control over hydrogel properties, such as porosity, pore size, and pore interconnectivity, remains a challenge. Traditional techniques for creating conventional crosslinked polymers have demonstrated limited success in the formation of hydrogels with large pore size, thus limiting cellular infiltration, tissue ingrowth, vascularization, and matrix mineralization (in the case of bone) of tissue-engineered constructs. Emerging technologies have demonstrated the ability to control microarchitectural features in hydrogels such as the creation of large pore size, porosity, and pore interconnectivity, thus allowing the creation of engineered hydrogel scaffolds with a structure and function closely mimicking native tissues. In this review, we explore the various technologies available for the preparation of macroporous scaffolds and their potential applications.
Keyword
MeSH Terms
Figure
Reference
-
1. Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006; 18:1345–1360.
Article2. Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev. 2010; 16:371–383.
Article3. Park S, Kim G, Jeon YC, Koh Y, Kim W. 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. J Mater Sci Mater Med. 2009; 20:229–234.
Article4. Kennedy S, Bencherif S, Norton D, Weinstock L, Mehta M, Mooney D. Rapid and extensive collapse from electrically responsive macroporous hydrogels. Adv Healthc Mater. 2013; 09. 12. [Epub]. http:dx.doi.org/10.1002/adhm.201300260.
Article5. Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, et al. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci U S A. 2012; 109:19590–19595.
Article6. Bencherif SA, Guillemot F, Huebsch N, Edwards DA, Mooney DJ. Cell-traction mediated configuration of the cell/extracellular-matrix interface plays a key role in stem cell fate. Med Sci (Paris). 2011; 27:19–21.7. Yoon JA, Bencherif SA, Aksak B, Kim EK, Kowalewski T, Oh JK, et al. Thermoresponsive hydrogel scaffolds with tailored hydrophilic pores. Chem Asian J. 2011; 6:128–136.
Article8. Cho HY, Gao H, Srinivasan A, Hong J, Bencherif SA, Siegwart DJ, et al. Rapid cellular internalization of multifunctional star polymers prepared by atom transfer radical polymerization. Biomacromolecules. 2010; 11:2199–2203.
Article9. Sun LT, Bencherif SA, Gilbert TW, Lotze MT, Washburn NR. Design principles for cytokine-neutralizing gels: Cross-linking effects. Acta Biomater. 2010; 6:4708–4715.
Article10. Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010; 9:518–526.
Article11. Bencherif SA, Washburn NR, Matyjaszewski K. Synthesis by AGET ATRP of degradable nanogel precursors for in situ formation of nanostructured hyaluronic acid hydrogel. Biomacromolecules. 2009; 10:2499–2507.
Article12. Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, Washburn NR, et al. Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials. 2009; 30:5270–5278.
Article13. Bencherif SA, Gao H, Srinivasan A, Siegwart DJ, Hollinger JO, Washburn NR, et al. Cell-adhesive star polymers prepared by ATRP. Biomacromolecules. 2009; 10:1795–1803.
Article14. Bencherif SA, Srinivasan A, Sheehan JA, Walker LM, Gayathri C, Gil R, et al. End-group effects on the properties of PEG-co-PGA hydrogels. Acta Biomater. 2009; 5:1872–1883.
Article15. Bencherif SA, Sheehan JA, Hollinger JO, Walker LM, Matyjaszewski K, Washburn NR. Influence of cross-linker chemistry on release kinetics of PEG-co-PGA hydrogels. J Biomed Mater Res A. 2009; 90:142–153.
Article16. Bencherif SA, Srinivasan A, Horkay F, Hollinger JO, Matyjaszewski K, Washburn NR. Influence of the degree of methacrylation on hyaluronic acid hydrogels properties. Biomaterials. 2008; 29:1739–1749.
Article17. Lin-Gibson S, Bencherif S, Antonucci JM, Jones RL, Horkay F. Synthesis and characterization of poly(ethylene glycol) dimethacrylate hydrogels. Macromol Symp. 2005; 227:243–254.
Article18. Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, et al. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004; 5:1280–1287.
Article19. Siegwart DJ, Srinivasan A, Bencherif SA, Karunanidhi A, Oh JK, Vaidya S, et al. Cellular uptake of functional nanogels prepared by inverse miniemulsion ATRP with encapsulated proteins, carbohydrates, and gold nanoparticles. Biomacromolecules. 2009; 10:2300–2309.
Article20. Siegwart DJ, Bencherif SA, Srinivasan A, Hollinger JO, Matyjaszewski K. Synthesis, characterization, and in vitro cell culture viability of degradable poly(N-isopropylacrylamide-co-5,6-benzo-2-methylene-1,3-dioxepane)-based polymers and crosslinked gels. J Biomed Mater Res A. 2008; 87:345–358.21. Ekaputra AK, Prestwich GD, Cool SM, Hutmacher DW. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (epsilon-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials. 2011; 32:8108–8117.
Article22. Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011; 63:300–311.
Article23. Street J, Bao M, deGuzman L, Bunting S, Peale FV Jr, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002; 99:9656–9661.
Article24. Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials. 2003; 24:181–194.
Article25. O'Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care. 2007; 15:3–17.26. Murphy CM, O'Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr. 2010; 4:377–381.
Article27. Murphy CM, O'Brien FJ, Little DG, Schindeler A. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013; 26:120–132.
Article28. Chen GP, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002; 2:67–77.
Article29. Battistella E, Varoni E, Cochis A, Palazzo B, Rimondini L. Degradable polymers may improve dental practice. J Appl Biomater Biomech. 2011; 9:223–231.
Article30. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005; 26:5474–5491.
Article31. Chirila TV, Constable IJ, Crawford GJ, Vijayasekaran S, Thompson DE, Chen YC, et al. Poly(2-hydroxyethyl methacrylate) sponges as implant materials: in vivo and in vitro evaluation of cellular invasion. Biomaterials. 1993; 14:26–38.
Article32. Gavrilas C, Han W, Li G, Omidian H, Rocca JG. Abbott Laboratories. Very-pure superporous hydrogels having outstanding swelling propertles. United States patent. WO/2009/029087. 2009. 03. 05.33. Huang X, Zhang Y, Donahue HJ, Lowe TL. Porous thermoresponsive-co-biodegradable hydrogels as tissue-engineering scaffolds for 3-dimensional in vitro culture of chondrocytes. Tissue Eng. 2007; 13:2645–2652.
Article34. Wei G, Ma PX. Partially nanofibrous architecture of 3D tissue engineering scaffolds. Biomaterials. 2009; 30:6426–6434.
Article35. Xu F, Sridharan B, Durmus NG, Wang S, Yavuz AS, Gurkan UA, et al. Living bacterial sacrificial porogens to engineer decellularized porous scaffolds. PLoS One. 2011; 6:e19344.
Article36. Stachowiak AN, Bershteyn A, Tzatzalos E, Irvine DJ. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv Mater. 2005; 17:399–403.
Article37. Draghi L, Resta S, Pirozzolo MG, Tanzi MC. Microspheres leaching for scaffold porosity control. J Mater Sci Mater Med. 2005; 16:1093–1097.
Article38. Davis HE, Leach JK. Hybrid and composite biomaterials for tissue engineering. In : Ashammakhi N, editor. Topics in multifunctional biomaterials and devices e-book. Davis (CA): Biomedical Engineering UC Davis;2008. p. 1–26.39. Capes JS, Ando HY, Cameron RE. Fabrication of polymeric scaffolds with a controlled distribution of pores. J Mater Sci Mater Med. 2005; 16:1069–1075.
Article40. Tran RT, Naseri E, Kolasnikov A, Bai X, Yang J. A new generation of sodium chloride porogen for tissue engineering. Biotechnol Appl Biochem. 2011; 58:335–344.
Article41. Qu T, Liu X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J Mater Chem B Mater Biol Med. 2013; 1:4764–4772.
Article42. Sultana N, Wang M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterisation of the scaffolds. J Mater Sci Mater Med. 2008; 19:2555–2561.
Article43. Sultana N, Wang M. PHBV/PLLA-based composite scaffolds containing nano-sized hydroxyapatite particles for bone tissue engineering. J Exp Nanosci. 2008; 3:121–132.
Article44. Thomson RC, Yaszemski MJ, Mikos AG. Polymer scaffold processing. In : Lanza RP, Langer R, Chick WL, editors. Principles of tissue engineering. Austin (TX): Academic Press;1997. p. 263–272.45. Hu Y, Grainger DW, Winn SR, Hollinger JO. Fabrication of poly(alpha-hydroxy acid) foam scaffolds using multiple solvent systems. J Biomed Mater Res. 2002; 59:563–572.
Article46. Pandit N, Malik R, Philips D. Tissue engineering: a new vista in periodontal regeneration. J Indian Soc Periodontol. 2011; 15:328–337.
Article47. Wang Y, Zhao Q, Hu Y, Sun L, Bai L, Jiang T, et al. Ordered nanoporous silica as carriers for improved delivery of water insoluble drugs: a comparative study between three dimensional and two dimensional macroporous silica. Int J Nanomedicine. 2013; 8:4015–4031.
Article48. Lytle JC, Stein A. Recent progress in syntheses and applications of inverse opals and related macroporous materials prepared by colloidal crystal templating. In : Cao G, Brinker CJ, editors. Annual reviews of nano research. New Jersey: World Scientific Publishing Co.;2006. p. 1–79.49. Zhang K, Yan H, Bell DC, Stein A, Francis LF. Effects of materials parameters on mineralization and degradation of sol-gel bioactive glasses with 3D-ordered macroporous structures. J Biomed Mater Res A. 2003; 66:860–869.
Article50. Zhang K, Simon CG Jr, Washburn NR, Antonucci JM, Lin-Gibson S. In situ formation of blends by photopolymerization of poly(ethylene glycol) dimethacrylate and polylactide. Biomacromolecules. 2005; 6:1615–1622.
Article51. Zhang K, Washburn NR, Simon CG Jr. Cytotoxicity of three-dimensionally ordered macroporous sol-gel bioactive glass (3DOM-BG). Biomaterials. 2005; 26:4532–4539.
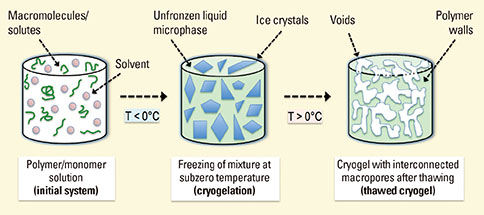
Article52. Henderson TM, Ladewig K, Haylock DN, McLean KM, O'Connor AJ. Cryogels for biomedical applications. J Mater Chem B Mater Biol Med. 2013; 1:2682–2695.
Article53. Plieva FM, Karlsson M, Aguilar MR, Gomez D, Mikhalovsky S, Galaev IY. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter. 2005; 1:303–309.
Article54. Lozinsky VI, Plieva FM, Galaev IY, Mattiasson B. The potential of polymeric cryogels in bioseparation. Bioseparation. 2001; 10:163–188.55. Plieva FM, Galaev IY, Mattiasson B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J Sep Sci. 2007; 30:1657–1671.
Article56. Dainiak MB, Galaev IY, Kumar A, Plieva FM, Mattiasson B. Chromatography of living cells using supermacroporous hydrogels, cryogels. In : Kumar A, Galaev IY, Mattiasson B, editors. Cell separation: fundamentals, analytical and preparative methods. Berlin: Springer;2007. p. 101–127.57. Hwang Y, Sangaj N, Varghese S. Interconnected macroporous poly(ethylene glycol) cryogels as a cell scaffold for cartilage tissue engineering. Tissue Eng Part A. 2010; 16:3033–3041.
Article58. Singh D, Tripathi A, Nayak V, Kumar A. Proliferation of chondrocytes on a 3-d modelled macroporous poly(hydroxyethyl methacrylate)-gelatin cryogel. J Biomater Sci Polym Ed. 2011; 22:1733–1751.
Article59. Mishra R, Goel SK, Gupta KC, Kumar A. Biocomposite cryogels as tissue engineered biomaterials for regeneration of critical-sized cranial bone defects. Tissue Eng Part A. 2013; 12. 11. [Epub]. http://dx.doi.org/10.1089/ten.tea.2013.0072.
Article60. Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006; 12:1197–1211.
Article61. Brown AN, Kim BS, Alsberg E, Mooney DJ. Combining chondrocytes and smooth muscle cells to engineer hybrid soft tissue constructs. Tissue Eng. 2000; 6:297–305.
Article62. Takei T, Sakai S, Ijima H, Kawakami K. Development of mammalian cell-enclosing calcium-alginate hydrogel fibers in a co-flowing stream. Biotechnol J. 2006; 1:1014–1017.
Article63. Tuzlakoglu K, Alves CM, Mano JF, Reis RL. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol Biosci. 2004; 4:811–819.
Article64. Vasilev MP, Volf LA, Kotetskin VV, Pukhova ZI, Meos AI. The spinning of collagen fibres. Fibre Chem. 1972; 4:48–50.
Article65. Lovett ML, Cannizzaro CM, Vunjak-Novakovic G, Kaplan DL. Gel spinning of silk tubes for tissue engineering. Biomaterials. 2008; 29:4650–4657.
Article66. Li D, Xia Y. Electrospinning of nanofibers: reinventing the wheel? Adv Mater. 2004; 16:1151–1170.
Article67. Zhong S, Zhang Y, Lim CT. Fabrication of large pores in electrospun nanofibrous scaffolds for cellular infiltration: a review. Tissue Eng Part B Rev. 2012; 18:77–87.
Article68. Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007; 59:1413–1433.
Article69. Sequeira SJ, Soscia DA, Oztan B, Mosier AP, Jean-Gilles R, Gadre A, et al. The regulation of focal adhesion complex formation and salivary gland epithelial cell organization by nanofibrous PLGA scaffolds. Biomaterials. 2012; 33:3175–3186.
Article70. Parrag IC, Zandstra PW, Woodhouse KA. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol Bioeng. 2012; 109:813–822.
Article71. Shabani I, Haddadi-Asl V, Seyedjafari E, Soleimani M. Cellular infiltration on nanofibrous scaffolds using a modified electrospinning technique. Biochem Biophys Res Commun. 2012; 423:50–54.
Article72. Li L, Qian Y, Jiang C, Lv Y, Liu W, Zhong L, et al. The use of hyaluronan to regulate protein adsorption and cell infiltration in nanofibrous scaffolds. Biomaterials. 2012; 33:3428–3445.
Article73. Baker BM, Gee AO, Metter RB, Nathan AS, Marklein RA, Burdick JA, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008; 29:2348–2358.
Article74. Leong MF, Rasheed MZ, Lim TC, Chian KS. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res A. 2009; 91:231–240.75. Lee YH, Lee JH, An IG, Kim C, Lee DS, Lee YK, et al. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials. 2005; 26:3165–3172.
Article76. Brown TD, Dalton PD, Hutmacher DW. Direct writing by way of melt electrospinning. Adv Mater. 2011; 23:5651–5657.
Article77. Leong MF, Chan WY, Chian KS, Rasheed MZ, Anderson JM. Fabrication and in vitro and in vivo cell infiltration study of a bilayered cryogenic electrospun poly(D,L-lactide) scaffold. J Biomed Mater Res A. 2010; 94:1141–1149.78. Jayasinghe SN, Irvine S, McEwan JR. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine (Lond). 2007; 2:555–567.
Article79. Townsend-Nicholson A, Jayasinghe SN. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules. 2006; 7:3364–3369.
Article80. Andreu N, Thomas D, Saraiva L, Ward N, Gustafsson K, Jayasinghe SN, et al. In vitro and in vivo interrogation of bio-sprayed cells. Small. 2012; 8:2495–2500.
Article81. Stankus JJ, Guan J, Fujimoto K, Wagner WR. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006; 27:735–744.
Article82. Dehghani F, Annabi N. Engineering porous scaffolds using gas-based techniques. Curr Opin Biotechnol. 2011; 22:661–666.
Article83. Keskar V, Marion NW, Mao JJ, Gemeinhart RA. In vitro evaluation of macroporous hydrogels to facilitate stem cell infiltration, growth, and mineralization. Tissue Eng Part A. 2009; 15:1695–1707.
Article84. Barbetta A, Barigelli E, Dentini M. Porous alginate hydrogels: synthetic methods for tailoring the porous texture. Biomacromolecules. 2009; 10:2328–2337.
Article85. Huang GY, Zhou LH, Zhang QC, Chen YM, Sun W, Xu F, et al. Microfluidic hydrogels for tissue engineering. Biofabrication. 2011; 3:012001.
Article86. Silva DS, Wallace DB, Cooley PW, Radulescu D, Hayes DJ. An inkjet printing station for neuroregenerative tissue engineering. In : Engineering in Medicine and Biology Workshop; 2007 Nov 11-12; Dallas (TX), USA. IEEE;2007. p. 71–73.87. Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, et al. Toward engineering functional organ modules by additive manufacturing. Biofabrication. 2012; 4:022001.
Article88. Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, et al. Rapid 3D printing of anatomically anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012; 4:035005.89. Pataky K, Braschler T, Negro A, Renaud P, Lutolf MP, Brugger J. Microdrop printing of hydrogel bioinks into 3D tissue-like geometries. Adv Mater. 2012; 24:391–396.
Article90. Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, et al. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnol Bioeng. 2010; 105:1178–1186.
Article91. Stampfl J, Schuster M, Baudis S, Lichtenegger H, Liska R. Biodegradable stereolithography resins with defined mechanical properties. In : Bartolo PJ, de Tecnologia e Gestao ES, editors. Virtual and rapid manufacturing. London: Taylor & Francis Group;2008. p. 283–286.92. Nguyen AK, Gittard SD, Koroleva A, Schlie S, Gaidukeviciute A, Chichkov BN, et al. Two-photon polymerization of polyethylene glycol diacrylate scaffolds with riboflavin and triethanolamine used as a water-soluble photoinitiator. Regen Med. 2013; 8:725–738.
Article93. Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009; 30:2724–2734.
Article94. Lo CW, Jiang H. Photopatterning and degradation study of dextran-glycidyl methacrylate hydrogels. Polym Eng Sci. 2009; 50:232–239.
Article95. Prado SS, Weaver JM, Love BJ. Gelation of photopolymerized hyaluronic acid grafted with glycidyl methacrylate. Mater Sci Eng C. 2011; 31:1767–1771.
Article96. Bartolo P. Stereolithography: materials, processes and applications. New York: Springer;2011.97. Jhaveri SJ, McMullen JD, Sijbesma R, Tan LS, Zipfel W, Ober CK. Direct three-dimensional microfabrication of hydrogels via two-photon lithography in aqueous solution. Chem Mater. 2009; 21:2003–2006.
Article98. Xue SH, Lv PJ, Wang Y, Zhao Y, Zhang T. Three dimensional bioprinting technology of human dental pulp cells mixtures. Beijing Da Xue Xue Bao. 2013; 45:105–108.99. Kim K, Lee CH, Kim BK, Mao JJ. Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res. 2010; 89:842–847.
Article100. Lee M, Wu BM. Recent advances in 3D printing of tissue engineering scaffolds. Methods Mol Biol. 2012; 868:257–267.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomimetic Polymer Scaffolds to Promote Stem Cell-Mediated Osteogenesis
- Development of Macroporous Chitosan Scaffolds for Eyelid Tarsus Tissue Engineering
- Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development
- The principles of artificial intelligence and its applications in dentistry
- Nanotechnology Biomimetic Cartilage Regenerative Scaffolds