Tuberc Respir Dis.
2007 Jun;62(6):499-505.
Diagnostic Utility of Pleural Fluid Soluble Triggering Receptor Expressed on Myeloid Cells 1 Protein in Patients with Exudative Pleural Effusion
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University College of Medicine, Seoul, Korea. jinhwalee@ewha.ac.kr
Abstract
- Background
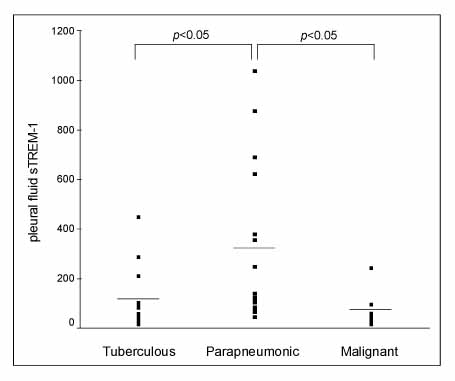
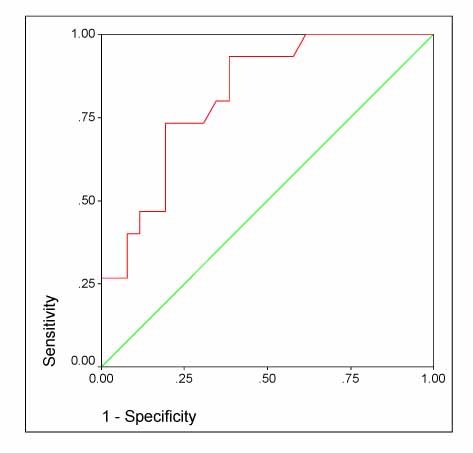
Triggering receptor expressed on myeloid cells 1 protein (TREM-1) is a cell surface molecule expressed on neutrophils and monocytes, and it plays an important role in myeloid cell-activated inflammatory response. The aim of this study was to investigate the diagnostic efficiency of soluble (s) TREM-1 in the patients who had pleural effusion from various causes. Methods: Forty-five patients with exudative pleural effusion were included in this study. The level of sTREM-1 was measured in both the serum and pleural fluids by immunoblot assay with using human-sTREM-1 antibody. Results: The pleural fluid sTREM-1 was significantly different in the three groups of exudative pleural effusion (p=0.011). Particularly, the patients with parapneumonic effusion were found to have significantly higher pleural fluid levels of sTREM-1 than patients with tuberculous (p<0.05) and malignant effusion, respectively (p<0.05). However, the serum sTREM-1 did not show a significant difference in the three groups. In order to evaluate the diagnostic utility of pleural fluid sTREM-1, the receiver operating characteristic (ROC) curve was constructed and the area under the curve (AUC) was 0.818 (p=0.001). Using a cutoff value of 103.5 pg/mL for the pleural fluid sTREM-1, the sensitivity and specificity were 73% and 81%, respectively, for differentiating parapneumonic effusion from tuberculous or malignant effusions. Conclusion: Pleural fluid sTREM-1 can be an additional marker for making the differential diagnosis of pleural effusion.
MeSH Terms
Figure
Reference
-
1. Light RW, Macgregor MI, Luchsinger PC, Ball WC Jr. Pleural effusions: the diagnostic sepreration of transudates and exudates. Ann Intern Med. 1972. 77:507–513.2. Marel M, Stastny B, Melinova L, Svandova E, Light RW. Diagnosis of pleural effusions. Experience with clinical studies, 1986 to 1990. Chest. 1995. 107:1598–1603.3. Bouchon A, Dietrich J, Colonna M. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol. 2000. 164:4991–4995.4. Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001. 410:1103–1107.5. Bouchon A, Hernandez-Munain C, Cella M, Colonna M. A DAP12-mediated pathway regulates expression of CC chemokine receptor 7 and maturation of human dendritic cells. J Exp Med. 2001. 194:1111–1122.6. Gibot S, Kolopp-Sarda MN, Béné MC, Bollaert PE, Lozniewski A, Mory F, et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004. 200:1419–1426.7. Bleharski JR, Kiessler V, Buonsanti C, Sieling PA, Stenger S, Colonna M, et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the Immune response. J immunol. 2003. 170:3812–3818.8. Gibot S, Cravoisy A, Levy B, Béné MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004. 350:451–458.9. Gibot S, Kolopp-Sarda MN, Béné MC, Cravoisy A, Levy B, Faure GC, et al. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med. 2004. 141:9–15.10. Nance KV, Shermer RW, Askim FB. Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991. 4:320–324.11. Light RW. Pleural diseases. 2001. 4th ed. Philadelphia: Lippincott Williams & Wilkins.12. Liu CL, Hsieh WY, Wu CL, Kuo HT, Lu YT. Triggering receptor expressed on myeloid cells-1 in pleural effusions: A marker of inflammatory disease. Respir Med. 2007. 101:903–909.13. Berger HW, Mejia E. Tuberculous pleurisy. Chest. 1973. 63:88–92.14. Valdes L, Alvarez D, San Jose E, Penela P, Valle JM, Garcia-Pazos JM, et al. Tuberculous pleurisy: a study of 254 patients. Arch Intern Med. 1998. 158:2017–2021.15. Yamamoto S, Dunn CJ, Willoughby DA. Studies on delayed hypersensitivity pleural exudates in guinea pigs. II. The interrelationship of monocytic and lymphocytic cells with respect to migration activity. Immunology. 1976. 30:513–519.16. Richeldi L, Mariani M, Losi M, Maselli F, Corbetta L, Buonsanti C, et al. Triggering receptor expressed on myeloid cells: role in the diagnosis of lung infections. Eur Respir J. 2004. 24:247–250.17. Plachouras D, Routsi C, Giamarellos-Bourboulis EJ, Spyridaki E, Andrianakis I, Metzelopoulos S, et al. Monocytes as a site of production of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in the septic host. Scand J Infect Dis. 2006. 38:909–915.18. Gibot S, Renard PE, Bollaert PE, Kolopp-Sarda MN, Béné MC, Faure GC, et al. Surface triggering receptor expressed on myeloid cells 1 expression patterns in septic shock. Intensive Care Med. 2005. 31:594–597.19. Gibot S, Cravoisy A, Kolopp-Sarda MN, Béné MC, Faure G, Bollaert PE, et al. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med. 2005. 33:792–796.20. Light RW, Erozan YS, Ball WC Jr. Cells in pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973. 132:854–860.21. Yam LT. Diagnostic significance of lymphocytes in pleural effusions. Ann Intern Med. 1967. 66:972–982.22. Light RW, Ball WC Jr. Glucose and amylase in pleural effusions. JAMA. 1973. 225:257–260.23. Lee JH, Chang JH. Diagnostic utility of serum and pleural fluid carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragments in patients with effusions from primary lung cancer. Chest. 2005. 128:2298–2303.24. Ocana I, Martinez-Vazquez JM, Segura RM, Fernandez-De-Sevilla T, Capdevila JA. Adenosine deaminase in pleural fluids. Test for diagnosis of tuberculous pleural effusion. Chest. 1983. 84:51–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Soluble Triggering Receptor Expressed on Myeloid cells-1: Role in the Diagnosis of Pleural Effusions
- Influence of Age on The Adenosine Deaminase Activity in Patients with Exudative Pleural Effusion
- Diagnostic Tools of Pleural Effusion
- Soluble Interleukin-2 Receptor (sIL-2R) Levels in Patients with Tuberculous and Malignant Pleural effusion
- Diagnostic Value of LDH and its Isoenzyme in Pleural Effusion