Restor Dent Endod.
2015 Nov;40(4):276-285. 10.5395/rde.2015.40.4.276.
Clinical and radiographical evaluation of mineral trioxide aggregate, biodentine and propolis as pulpotomy medicaments in primary teeth
- Affiliations
-
- 1Department of Pedodontics and Preventive Dentistry, Maulana Azad Institute of Dental Sciences, MAMC complex, BSZ Marg, New Delhi, India. kusumbharti1984@gmail.com
- 2Department of Pediatric and Preventive Dentistry, Faculty of Dental Sciences, King George Medical University, Lucknow, India.
- KMID: 2316947
- DOI: http://doi.org/10.5395/rde.2015.40.4.276
Abstract
OBJECTIVES
The purpose of this study was to evaluate the efficacy of mineral trioxide aggregate (MTA), Biodentine and Propolis as pulpotomy medicaments in primary dentition, both clinically and radiographically.
MATERIALS AND METHODS
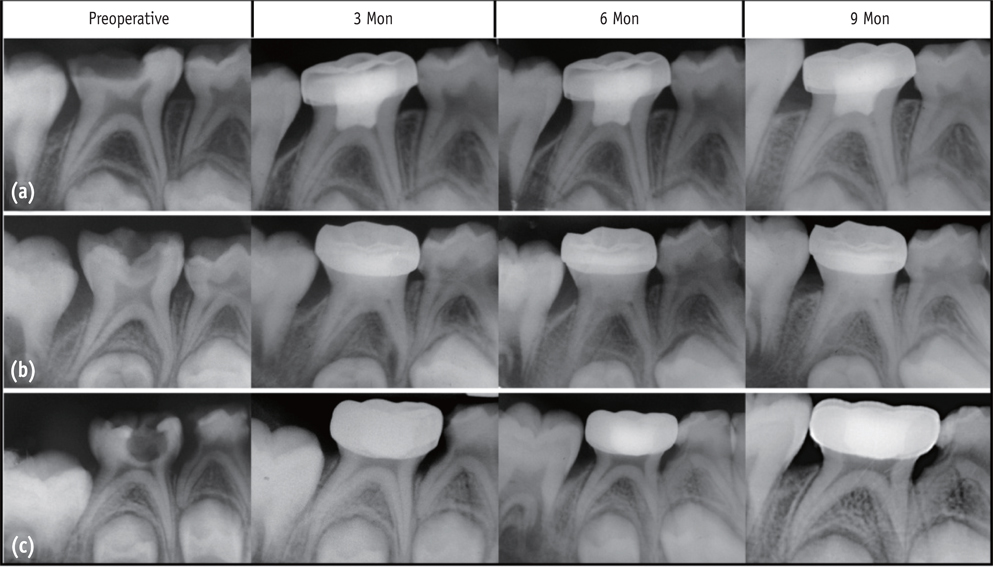
A total of 75 healthy 3 to 10 yr old children each having at least one carious primary molar tooth were selected. Random assignment of the pulpotomy medicaments was done as follows: Group I, MTA; Group II, Biodentine; Group III, Propolis. All the pulpotomized teeth were evaluated at 3, 6, and 9 mon clinically and radiographically, based on the scoring criteria system.
RESULTS
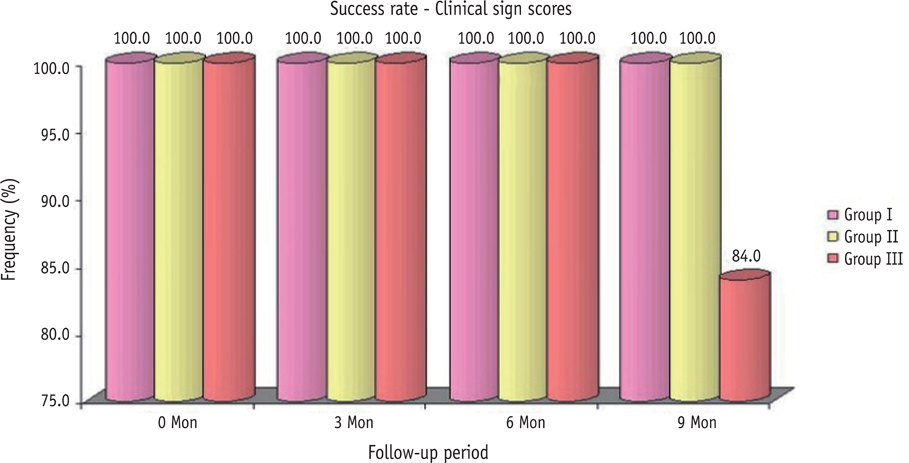
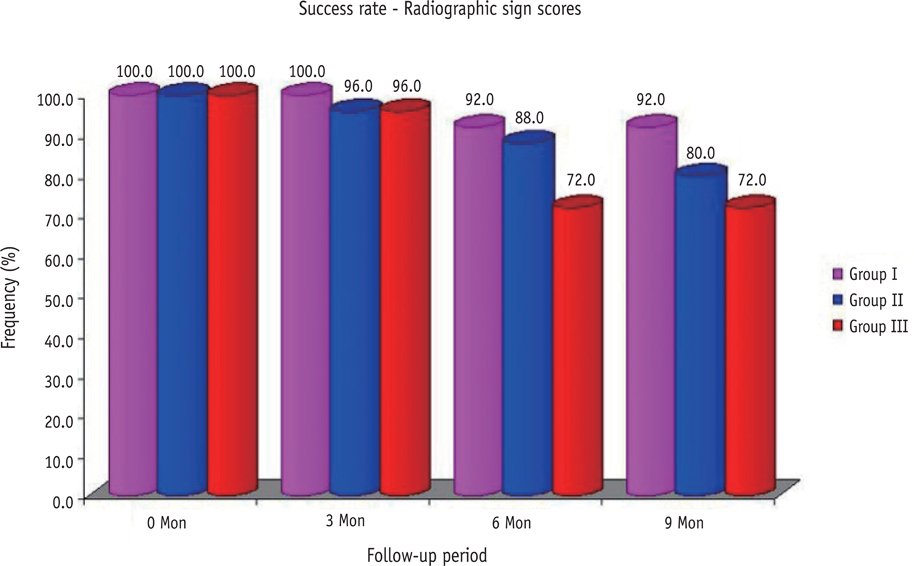
The clinical success rates were found to be similar among the three groups at 3 and 6 mon where as a significant decrease in success rate was observed in Group III (84%) compared to both Group I (100%) and Group II (100%) at 9 mon. Radiographic success rates over a period of 9 mon in Groups I, II, and III were 92, 80, and 72%, respectively.
CONCLUSIONS
Teeth treated with MTA and Biodentine showed more favorable clinical and radiographic success as compared to Propolis at 9 mon follow-up.
MeSH Terms
Figure
Reference
-
1. Caicedo R, Abbott PV, Alongi DJ, Alarcon MY. Clinical, radiographic and histological analysis of the effects of mineral trioxide aggregate used in direct pulp capping and pulpotomies of primary teeth. Aust Dent J. 2006; 51:297–305.
Article2. Magnusson B. Attempts to predict prognosis of pulpotomy in primary molars. Scand J Dent Res. 1970; 78:232–240.3. Ranly DM. Pulpotomy therapy in primary teeth: new modalities for old rationales. Pediatr Dent. 1994; 16:403–409.4. Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatr Dent. 2001; 23:15–18.5. Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynolds KE. Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc. 1978; 96:805–813.
Article6. Primosch RE, Glomb TA, Jerrell RG. Primary tooth pulp therapy as taught in Predoctoral pediatric dental programs in the United States. Pediatr Dent. 1997; 19:118–122.7. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999; 25:197–205.
Article8. Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005; 27:129–136.9. Oguntebi BR, Heaven T, Clark AE, Pink FE. Quantitative assessment of dentin bridge formation following pulpcapping in miniature swine. J Endod. 1995; 21:79–82.
Article10. Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996; 127:1491–1494.
Article11. Damamaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005; 21:731–738.
Article12. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part I: chemical, physical, and antibacterial properties. J Endod. 2010; 36:16–27.
Article13. Laurent P, Camps J, About I. Biodentine induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012; 45:439–448.
Article14. Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, Zhu Y. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011; 37:1240–1246.
Article15. Tran XV, Gorin C, Willing C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calicum-silicate-based restorative cement on pulp repair. J Dent Res. 2012; 91:1166–1171.
Article16. Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012; 38:1220–1226.
Article17. Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001; 15:561–571.
Article18. Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem Toxicol. 1998; 36:347–363.
Article19. Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995; 26:83–99.
Article20. Al-Haj Ali SN. In vitro toxicity of propolis in comparison with other primary teeth pulpotomy agents on human fibroblasts. J Investig Clin Dent. 2015; 04. 27. DOI: 10.1111/jicd.12157. [Epub ahead of print].21. Ozório JE, Carvalho LF, de Oliveira DA, de Sousa-Neto MD, Perez DE. Standardized propolis extract and calcium hydroxide as pulpotomy agents in primary pig teeth. J Dent Child (Chic). 2012; 79:53–58.22. Parolia A, Kundabala M, Rao NN, Acharya SR, Agrawal P, Mohan M, Thomas M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust Dent J. 2010; 55:59–64.
Article23. Zurn D, Seale NS. Light-cured calcium hydroxide vs formocresol in human primary molar pulpotomies: a randomized controlled trial. Pediatr Dent. 2008; 30:34–41.24. Sushynski JM, Zealand CM, Botero TM, Boynton JR, Majewski RF, Shelburne CE, Hu JC. Comparison of gray mineral trioxide aggregate and diluted formocresol in pulpotomized primary molars: a 6- to 24-month observation. Pediatr Dent. 2012; 34:120–128.25. Scheller S, Ilewicz L, Luciak M, Skrobidurska D, Stojko A, Matuga W. Biological properties and chemical application of propolis. IX. Experimental observation on the influence of ethanol extract of propolis (EEP) on dental pulp regeneration. Arzneimittelforschung. 1978; 28:289–291.26. Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013; 39:743–747.
Article27. Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002; 50:2502–2506.
Article28. Croll TP, Killian CM. Zinc oxide-eugenol pulpotomy and stainless steel crown restoration of a primary molar. Quintessence Int. 1992; 23:383–388.29. Gruythuysen RJ, Weerheijm KL. Calcium hydroxide pulpotomy with a light-cured cavity-sealing material after two years. ASDC J Dent Child. 1997; 64:251–253.30. Zealand CM, Briskie DM, Botero TM, Boynton JR, Hu JC. Comparing gray mineral trioxide aggregate and diluted formocresol in pulpotomized human primary molars. Pediatr Dent. 2010; 32:393–399.31. Erdem AP, Guven Y, Balli B, Ilhan B, Sepet E, Ulukapi I, Aktoren O. Success rates of mineral trioxide aggregate, ferric sulfate, and formocresol pulpotomies: a 24-month study. Pediatr Dent. 2011; 33:165–170.32. Willard RM. Radiographic changes following formocresol pulpotomy in primary molars. ASDC J Dent Child. 1976; 43:414–415.33. Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: long-term follow up. Pediatr Dent. 1997; 19:327–330.34. Koo H, Gomes BP, Rosalen PL, Ambrosano GM, Park YK, Cury JA. In vitro antimicrobial activity of propolis and arnica montana against oral pathogens. Arch Oral Biol. 2000; 45:141–148.
Article35. Silva FB, Almeida JM, Sousa SM. Natural medicaments in endodontics - a comparative study of the antiinflammatory action. Braz Oral Res. 2004; 18:174–179.
Article36. Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, Endo Y, Sato T, Satoh S, Tadano T. Antiinflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull. 2006; 29:96–99.
Article37. Jabbarifar SE, Khademi AA, Ghasemi D. Success rate of formocresol pulpotomy versus mineral trioxide aggregate in human primary molar tooth. J Res Med Sci. 2004; 9:304–307.38. Sonmez D, Sari S, Cetinbaş T. A Comparison of four pulpotomy techniques in primary molars: a long-term follow-up. J Endod. 2008; 34:950–955.
Article39. del Carmen González Rodríguez W, Carpio MHC, Ramos MRM, Milanés MG, Antúnez LN. Pulpotomies of dead pulps in temporal molars using 10% propolis tinction. Rev Cubana Estomatol [online]. 2007; 44(3):Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0034-75072007000300006&lng=en&nrm=iso&tlng=en (updated 2015 Sep 3).40. Waterhouse PJ, Nunn JH, Whitworth JM. Primary molar vital pulp theory. Br Dent J. 2000; 188:417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of mineral trioxide aggregate in the treatment of horizontal root fracture with a 4-year follow-up: case report
- The push-out bond strength of BIOfactor mineral trioxide aggregate, a novel root repair material
- Status and Survey of Pulp Treatment by Korean Pediatric Dentists
- Push-out bond strength and marginal adaptation of apical plugs with bioactive endodontic cements in simulated immature teeth
- Osteomyelitis on Maxilla Caused by Arsenic Trioxide