Nutr Res Pract.
2007 Dec;1(4):260-265.
Influence of preserved brewing yeast strains on fermentation behavior and flocculation capacity
- Affiliations
-
- 1Department of Fermented Food Science, Seoul University of Venture and Information, Seoul 137-070, Korea. sakang@suv.ac.kr
- 2Department of Biotechnology, Technical University of Berlin, Berlin, Germany.
Abstract
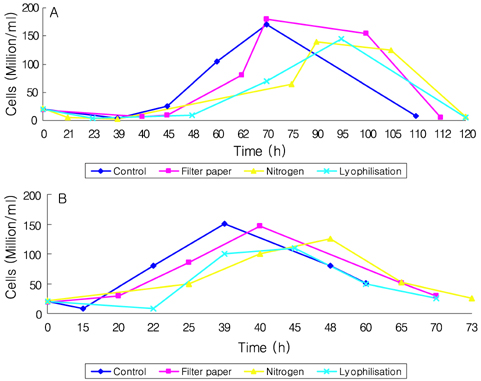
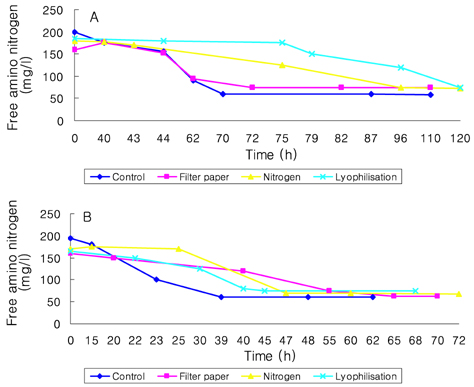
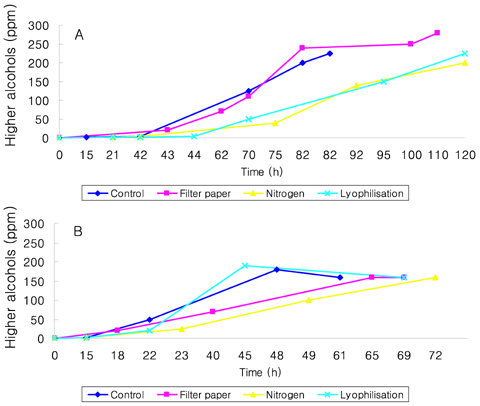
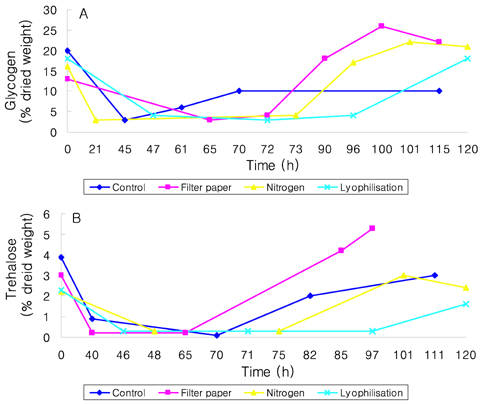
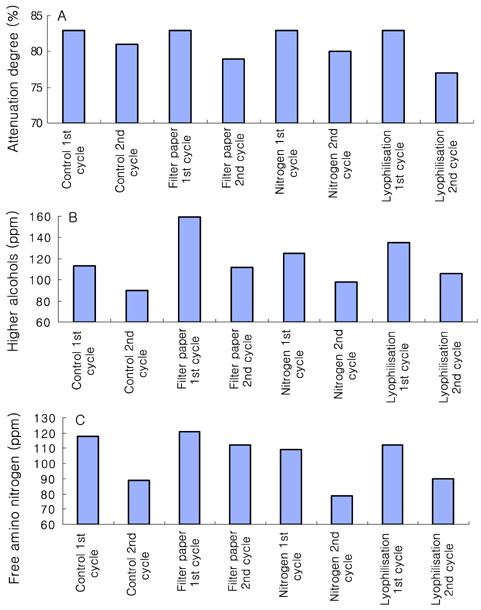
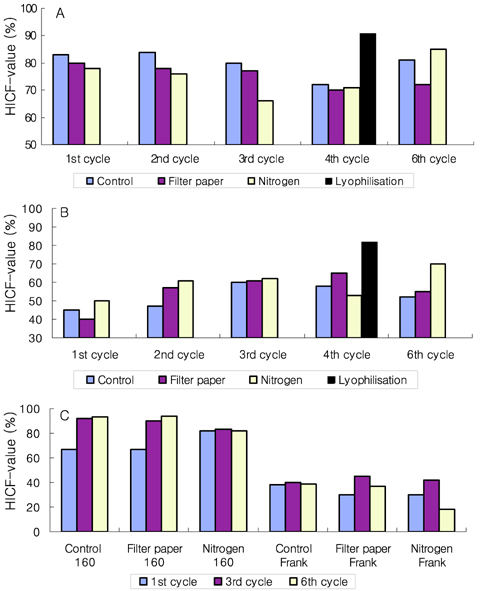
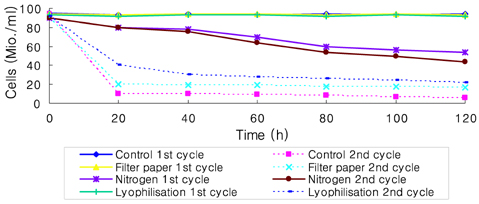
- Preservation methods on the physiological and brewing technical characters in bottom and top brewing yeast strains were investigated. The preserved yeasts were reactivated after 24 months storage and grown up to stationary phase. The samples of filter paper storage indicated a higher cell growth and viability during propagation than those of nitrogen and lyophilization storage independent on propagation temperature. In addition, the filter paper storage demonstrated a faster absorption of free amino nitrogen and a highest level of higher aliphatic alcohols production during propagation than other preservation methods, which can be attributed to intensive cell growth during propagation. Moreover, the filter paper storage showed a faster accumulation for glycogen and trehalose during propagation, whereas, in particular, lyophilization storage noted a longer adaptation time regarding synthesis of glycogen and trehalose with delayed cell growth. In beer analysis, the filter paper storage formed an increased higher aliphatic alcohols than control. In conclusion, the preservation of filter paper affected positively on yeast growth, viability and beer quality independent on propagation temperature. In addition, in this study, it was obtained that the HICF and Helm-test can be involved as rapid methods for determination of flocculation capacity.
MeSH Terms
Figure
Reference
-
1. Amory DE, Rouxhet PG, Dufour JP. Flocculence of brewery yeasts and their surface properties: chemical composition, electrostatic charge and hydrophobicity. J Inst Brew. 1988. 94:79–84.
Article2. Costa MJ, Ferreira PMS. Cerevisiae flocculation: identification of specific cell wall proteins. European Brewery Convention Congress. 1993. Oslo: 283–290.3. Dengis PB, Nelissen LR, Rouxhet PG. Mechanisms of yeast flocculation: comparison of top and bottom fermenting strains. Appl Environ Microbiol. 1995. 61:718–728.
Article4. Dengis PB, Rouxhet PG. Surface properties of top and bottom fermenting yeast. Yeast. 1997. 13:931–943.
Article5. Dengis PB, Rouxhet PG. Flocculation mechanisms of top and bottom fermenting brewing yeast. J Inst Brew. 1997. 103:257–261.
Article6. Fischborn T. Untersuchungen zum Trocknungsverhalten untergaeriger Hefen. 1997. Technische Universitaet Muenchen;Ph.D. Disser.7. Fischer K, Rahn J. Einfluss der Stammkonservierung auf die genetische Satabilitaet von Backhefen. Branntweinwirtschfaft. 2000. 140:17–21.8. Fontana A, Bidenne C, Ghommidh C, Guiraud P, Vezinhet F. Study of the flocculation of Saccharomyces diastaticus NCYC 625. J Inst Brew. 1992. 98:401–407.9. Harrison M. Laboratory management of yeast. Ferment. 1996. 9:35–36.10. Hill LR. Norris JR, Richmond MH, editors. Preservation of microorganisms. Essays in Appl Microbiol. 1981. New York, London, Sydney, Toronto: John Wiley, Sons Ltd..11. Jibiki MA, Ishibiki T, Yamashita H, Eto M. A rapid and simple assay to measure flocculation in brewer's yeast. The Masters Brewers Association of the Americans Technical Quarterly. 1997. 34:278–281.12. Kamada K, Murata M. On the mechanism of brewer's yeast flocculation. Agric Biol Chem. 1984. 48:2423–2433.
Article13. Pfenninger H. Brautechnische analysenmethoden Band II. Selbstverlag der Mitteleuropaeische Brautechnische Analysenkommission. 1993. 3rd Edition. Freising: Auflage.14. Quain DE, Tubb RS. The importance of glycogen in brewing yeasts. The Masters Brewers Association of the Americans Technical Quarterly. 1982. 19:29–33.15. Romano P, Suzzi G, Vannini L. Relationship between foaming and flocculence in Saccharomyces cerevisiae wine yeasts. Colloids Surf B Biointerfaces. 1994. 2:511–515.
Article16. Rouxhet PG, Mozes N, Dengis PB, Dufrene YF, Gerin PA, Genet MJ. Application of X-ray photoelection spectroscopy to microorganisms. Colloids Surf B Biointerfaces. 1994. 2:347–369.17. Sips R. Quantifizierung und Identifizierung von Starterkulturen nach morphologischen und genetischen Kriterien. 1998. Technische Universitaet Berlin;Ph.D. Disser.18. Smit G, Straver MH, Lugtenberg BJJ, Kijne JW. Flocculence of Saccharomyces cerevisiae cells is induced by nutrient limitation, with cell surface hydrophobicity as a major determinant. Appl Environ Microbiol. 1992. 58:3709–3714.
Article19. Soares EV, Mota M. Quantification of yeast flocculation. J Inst Brew. 1997. 103:93–98.
Article20. Straver MH, Smit G, Kjine JW. Induced cell surface hydrophobicity influences of brewer's yeast during fermentation in wort. Yeast. 1994. 9:527–532.
Article21. Straver MH, Smit G, Kjine JW. Isolation and partial characterization of a mannose specific agglutinin from brewer's yeast involved in flocculation. Yeast. 1994. 10:1183–1193.
Article22. Stewart RJ, Russel I, Stewart GG. Characterization of affinity-purified cell wall-binding proteins of Saccharomyces cerevisiae: possible role in flocculation. American Society of Brewing Chemists. 1995. 53:111–116.
Article23. Thorne RSW, Nohr B. Some observation on the stability of a brewing yeast strain. The Brewers Digest. 1963. 38:36–39.24. Tybussek R, Linz F, Schuegerl K, Mozes N, Leonard AJ, Rouxhet PG. Comparison of the continuous flotation performances of Saccharomyces cerevisiae LBG H620 and DSM 2155 strains. Appl Microbiol Biotechnol. 1994. 41:13–22.
Article25. Van Hamersveld EH, Van der Lans RGJM, Caulet PJC, Luyben KChAM. Modeling brewer's yeast flocculation. Biotechnol Bioeng. 1998. 57:331–341.
Article26. Wackerbauer K, Tayama T, Fitzner M, Kunerth S. Zeitgemaesses management der Anstellhefe. Brauwelt. 1997a. 3:80–87.27. Wackerbauer K, Tayama T, Kunerth S. Neurere Erkenntnisse des Einflusses der Hefelagerung auf die Gaeaktivitaet und Vitalitaet von Hefen in nachfolgenden Gaerungen. Monatsschrift fuer Brauwissenschaft. 1997b. 50:132–137.28. Winkler K, Kienle I, Burgert M, Wagner JC, Holzer H. Metabolic regulation of the trehalose content of vegetative yeast. FEBS Lett. 1991. 291:269–272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microbiological Characteristics of Wild Yeast Strain Pichia anomala Y197-13 for Brewing Makgeolli
- LAMMER Kinase Lkh1 Is an Upstream Regulator of Prk1-Mediated Non-Sexual Flocculation in Fission Yeast
- Identification of Wild Yeast Strains and Analysis of Their beta-Glucan and Glutathione Levels for Use in Makgeolli Brewing
- Screening of Functional Rhizopus stolonifer for Alcohol Fermentation and Production of High Quality Korean Traditional Rice Wine
- Metabolite Profiling during Fermentation of Makgeolli by the Wild Yeast Strain Saccharomyces cerevisiae Y98-5