Nutr Res Pract.
2007 Sep;1(3):189-194.
The antioxidant and cytotoxic activities of Sonchus oleraceus L. extracts
- Affiliations
-
- 1School of Biotechnology, Kangwon National University, Chuncheon, Kangwon-Do 200-701, Korea. mhwang@kangwon.ac.kr
Abstract
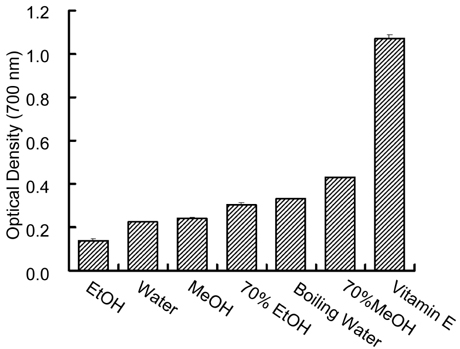
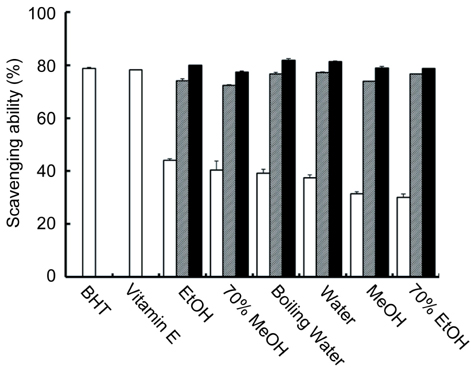
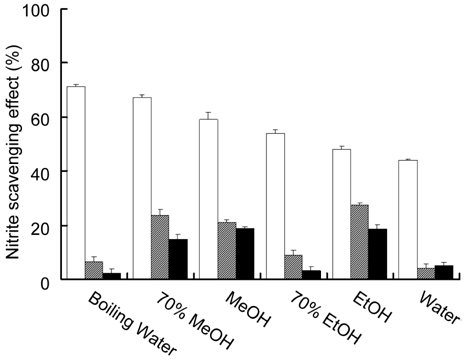
- This study investigated in vitro antioxidant activity of Sonchus oleraceus L. by extraction solvent, which were examined by reducing power, hydroxyl radical-scavenging activity(HRSA) and 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assays. 70% MeOH extract had the greatest reducing power while EtOH extract had the greatest HRSA. The antioxidant activity of S. oleraceus extracts was concentration dependent and its IC50 values ranged from 47.1 to 210.5 microgram/ml and IC50 of 70% MeOH, boiling water and 70% EtOH extracts were 47.1, 52.7 and 56.5 microgram/ml, respectively. 70% MeOH extract of S. oleraceus contained the greatest amount of both phenolic and flavonoid contents. The extracts tested had greater nitrite scavenging effects at lower pH conditions. The cytotoxic activity showed that EtOH extract had the best activity against the growth of stomach cancer cell. These results suggest that S. oleraceus extract could be used as a potential source of natural antioxidants.
MeSH Terms
Figure
Reference
-
1. Ames B. Micronutrients prevent cancer and delay aging. Toxicology letters. 1998. 102:5–18.
Article2. Aruoma OI. Deoxyribose assay for detecting hydroxyl radicals. Methods in Enzymology. 1994. 233:57–66.3. Aziz T, Mehmet ED, Nazime M, Ibrahim K, Kudret G. Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem. 2007. 101:267–273.4. Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004. 74:2157–2184.
Article5. Cakir A, Mavi A, Yildirim A, Duru ME, Harmandar M, Kazaz C. Isolation and characterization of antioxidant phenolic compounds from the aerial parts of Hypericum hyssopifolium L. by activity-guided fractionation. J Ethnopharmacol. 2003. 87:73–83.
Article6. Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis. 2002. 10:178–182.7. Das NP, Pereira TA. Effects of flavonoids on thermal autoxidation of palm oil: structure-activity relationships. J Am Oil Chem Soc. 1990. 67:255–258.
Article8. Decker EA. Phenolics: prooxidants or antioxidants? Nutr Rev. 1997. 55:396–407.
Article9. Duh PD. Antioxidant activity of burdock (Arctium lappa Linne): its scavenging effect on free radical and active oxygen. J Am Oil Chem Soc. 1998. 75:455–461.
Article10. Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harnjyur (Chyrsanthemum morifolium Ramat). Swiss Society of Food Science and Technology. 1999. 3:269–277.11. Gordon MH. Hudson BJF, editor. The mechanism of antioxidant action in vitro. Food Antioxidants. 1990. London. UK: Elsevier;1–18.
Article12. Guil-Guerrero JL, Giménez-Giménez A, Rodríguez-García I, Torija-Isasa ME. Nutritional composition of Sonchus species (A. asper L, S. oleraceus L and S. tenerrimus L). J Sci Food Agric. 1998. 76:628–232.13. Halliwell B. Antioxidant characterization: methodology and mechanism. Biochem Pharmacol. 1995. 49:1341–1348.14. Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants and human disease: Where are we now? J Lab Clin Med. 1992. 119:598–620.15. Holm LG, Plucknett DL, Pancho JV, Herberger JP. The world's worst weeds: Distribution and Biology. 1977. Honolulu: University Press of Hawaii;436–439.16. Howard LR, Pandjaitan N, Morelock T, Gil MI. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J Agric Food Chem. 2002. 50:5891–5896.
Article17. Huxley RR, Neil H. The relationship between dietary flavonol intake and coronary heart disease mortality: a metaanalysis of prospective cohort studies. Eur J Clin Nutr. 2003. 57:904–908.
Article18. Ito N, Fukushima S, Hasegawa A, Shibata M, Ogiso T. Carcinogenecity of butylated hydroxylanisole in F 344 rats. J Natl Cancer Inst. 1983. 70:343–347.19. Jeon TW, Jo CH, Kim KH, Byun MW. Inhibitory effect on tyrosinase and xanthine oxidase, and nitrite scavenging activities of Schizandrae Fructus extract by gamma irradiation. Korean Journal of Food Preservation. 2002. 9:369–374.20. Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics, and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999. 47:4638–4644.
Article21. Kang YH, Park YK, Lee GD. The nitrite scavenging and electron donating ability of phenolic compounds. Korean Journal of Food Science and Technology. 1996. 28:232–239.22. Kato FT, Puck TT. Mutagenesis by carcinogenic nitroso compounds. J Cell Physiol. 1971. 78:139–144.23. Kilani S, Ammar RB, Bouhlel I, Abdelwahed A, Hayder N, Mahmoud A, Ghedira K, Chekir-Ghedira L. Investigation of extracts from (Tunisian) Cyperus rotundus as antimutagens and radical scavengers. Environ Toxicol Pharmacol. 2005. 20:478–484.
Article24. Komali AS, Zheng Z, Shetty K. A mathematical model for the growth kinetics and synthesis of phenolics in oregano (Origanum vulgare) shoot cultures inoculated with Pseudomonas species. Process Biochem. 1999. 35:227–235.
Article25. Ma M, Hong CL, An SQ, Li B. Seasonal, spatial, and interspecific variation in quercetin in apocynum venetum and poacynum hendersonii, Chinese traditional herbal teas. J Agric Food Chem. 2003. 51:2390–2393.
Article26. Moller JKS, Madsen HL, Altonen T, Skibsted LH. Dittany (Origanum dictamnus) as a source of water-extractable antioxidants. Food Chem. 1999. 64:215–219.
Article27. Noh KS, Yang MO, Cho EJ. Nitrite scavenging effect of Umbelligeraeceae. Korean Journal of Food and Cookery Science. 2002. 18:8–12.28. Ordonez AAL, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (jacq.) Swartz extracts. Food Chem. 2006. 97:452–458.29. Osawa T. Novel Natural Antioxidants for Utilization in Food and Biological Systems. 1994. Tokyo. Japan: Japan Scientific Scoieties Press;241–251.30. Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese Journal of Nutrition. 1986. 44:307–315.
Article31. Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002. 50:81–86.
Article32. Siriwardhana N, Lee KW, Kim SH, Ha WJ, Jeon YJ. Antioxidant activity of Hizikia fusiformis on reactive oxygen scavenging and lipid peroxidation inhibition. Food Science and Technology International. 2003. 9:339–346.
Article33. Soares JR, Dins TCP, Cunha AP, Ameida LM. Antioxidant activity of some extracts of Thymus zygis. Free Radic Res. 1997. 26:469–478.34. Sun HX, Qin F, Pan YJ. In vitro and in vivo immunosuppressive activity of Spica prunellae ethanol extract on the immune responses in mice. J Ethnopharmacol. 2005. 101:31–36.
Article35. Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998. 46:4113–4117.
Article36. Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995. 43:27–32.
Article37. Zheng W, Wang SY. Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem. 2001. 49:5165–5170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of In vitro Antioxidant, Antidiabetic and Cytotoxic Activities of Sphaeranthus africanus Extracts
- Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots
- Antioxidant activity of ethanol extract of Lycium barbarum's leaf with removal of chlorophyll
- Phytochemical Characterization of Vitex negundo Leaves: a Potent Antiandrogenic and Antioxidant Agent
- In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweet potato