Nutr Res Pract.
2007 Mar;1(1):29-35.
Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells
- Affiliations
-
- 1Department of Food and Nutrition, Andong National University, Gyeongbuk 760-749, Korea. iskwun@andong.ac.kr
- 2Center of Scientific Instruments, Andong National University, Gyeongbuk 760-749, Korea.
- 3Department of Food Science and Biotechnology, Andong National University, Gyeongbuk 760-749, Korea.
- 4Cellular Integrity Division, Rowett Research Institute, Aberdeen AB21 9SB, Scotland, UK.
Abstract
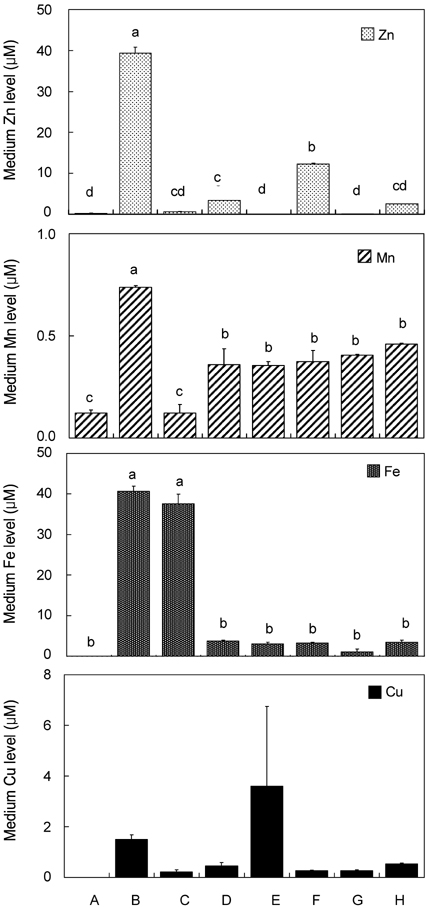
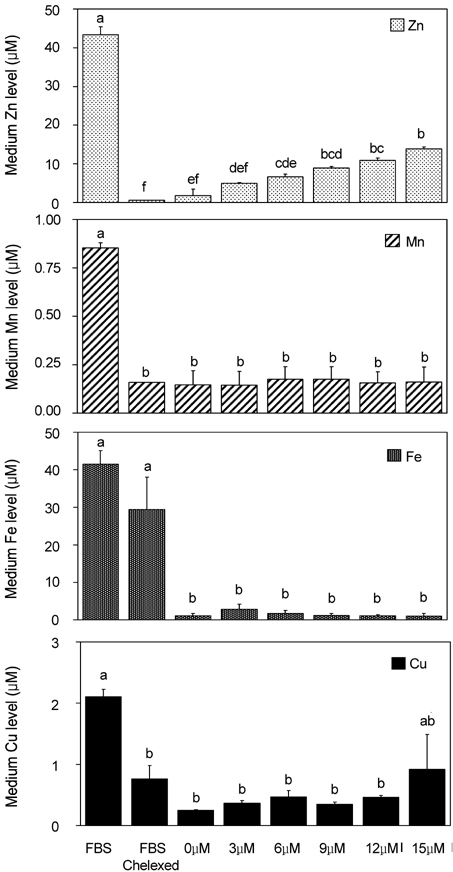
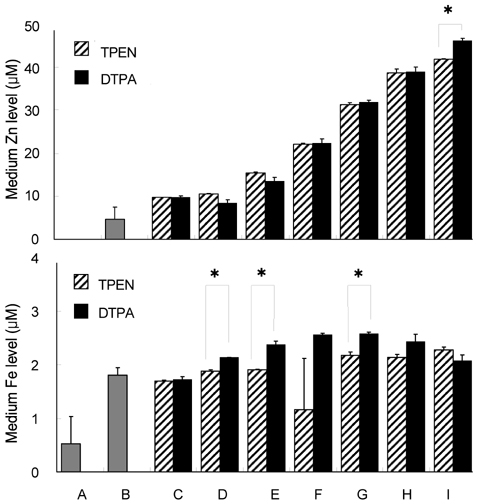
- Trace mineral studies involving metal ion chelators have been conducted in investigating the response of gene and protein expressions of certain cell lines but a few had really focused on how these metal ion chelators could affect the availability of important trace minerals such as Zn, Mn, Fe and Cu. The aim of the present study was to investigate the availability of Zn for the treatment of MC3T3-E1 osteoblast-like cells and the availability of some trace minerals in the cell culture media components after using chelexing resin in the FBS and the addition of N,N,N',N'-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN, membrane-permeable chelator) and diethylenetriaminepentaacetic acid (DTPA, membrane-impermeable chelator) in the treatment medium. Components for the preparation of cell culture medium and Zn-treated medium have been tested for Zn, Mn, Fe and Cu contents by atomic absorption spectrophotometer or inductively coupled plasma spectrophotometer. Also, the expression of bone-related genes (ALP, Runx2, PTH-R, ProCOL I, OPN and OC) was measured on the cellular Zn depletion such as chelexing or TPEN treatment. Results have shown that using the chelexing resin in FBS would significantly decrease the available Zn (p<0.05) (39.4 +/- 1.5 micrometer vs 0.61 +/- 10.15 micrometer) and Mn (p<0.05) (0.74 +/- 0.01 micrometer vs 0.12 +/- 0.04 micrometer). However, levels of Fe and Cu in FBS were not changed by chelexing FBS. The use of TPEN and DTPA as Zn-chelators did not show significant difference on the final concentration of Zn in the treatment medium (0, 3, 6, 9, 12 micrometer) except for in the addition of higher 15 micrometer ZnCl2 which showed a significant increase of Zn level in DTPA-chelated treatment medium. Results have shown that both chelators gave the same pattern for the expression of the five bone-related genes between Zn- and Zn+, and TPEN-treated experiments, compared to chelex-treated experiment, showed lower bone-related gene expression, which may imply that TPEN would be a stronger chelator than chelex resin. This study showed that TPEN would be a stronger chelator compared to DTPA or chelex resin and TPEN and chelex resin exerted cellular zinc depletion to be enough for cell study for Zn depletion.
Keyword
MeSH Terms
Figure
Reference
-
1. Aballay A, Sarrouf MN, Colombo MI, Stahl PD, Mayorga LS. Zn2+ depletion blocks endosome fusion. Biochem J. 1995. 312:919–923.2. Aisen P, Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980. 49:357–393.
Article3. Avery RA, Bettger WJ. Zinc deficiency alters the protein composition of the membrane skeleton but not the extratability of oligomeric form of spectrin in rat erythrocyte membrane. J Nutr. 1992. 122:428–434.
Article4. Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990. 8:281–291.
Article5. Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001. 14:331–341.
Article6. Chattopadhyay S, Freake HC. Zinc chelation enhances thyroid hormone induction of growth hormone mRNA in GH3 cells. Mol Cell Endocrinol. 1998. 136:151–157.
Article7. Chesters JK, Petrie L, Vint H. Specificity and timing of the Zn2+ requirement for DNA synthesis by 3T3 cells. Exp Cell Res. 1989. 184:499–508.
Article8. Cho YE, Lomeda RAR, Kim YH, Ryu SH, Choi JY, Kim HJ, Beattie JH, Kwun IS. Zinc deficiency decreased alkaline phosphatase expression and bone matrix Ca deposits in osteoblast-like MC3T3-E1 cells. Nutritional Sciences. 2005. 8:205–211.9. Cinatl J Jr, Hoffmann F, Cinatl J, Weber B, Scholz M, Rabenau H, Stieneker F, Kabickova H, Blasko M, Doerr HW. Invitro inhibition of human cytomegalovirus replication by calcium trinatrium diethylenetriaminepentaacetic acid. Antiviral Res. 1996. 31:23–24.10. Frederickson CJ, Suh SW, Koh JY, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002. 50:1659–1662.
Article11. Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cutured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001. 42:460–465.12. Kanekiyo M, Itoh N, Mano M, Kawasaki A, Tanaka J, Muto N, Tanaka K. Cellular zinc status regulates cytomegalovirus major immediate-early promoter. Antiviral Res. 2000. 47:207–214.
Article13. Lefebvre D, Beckers F, Ketelslegers JM, Thissen JP. Zinc regulation of insulin-like growth factor-I (IGF-I), growth hormone receptor (GHR) and binding protein (GHBP) gene expression in rat cultured hepatocytes. Mol Cell Endocrinol. 1998. 138:127–136.
Article14. Libin C, Schoene NW, Zhu L, Fanzo J, Alshatwi A, Lei KY. Zinc depletion reduced Egr-1 and HNF-3β expression and apolipoprotein A-I promoter activity in Hep G2 cells. Am J Physiol Cell Physiol. 2002. 283:C623–C630.15. Nakatini T, Kennedy DO, Murakami Y, Yano Y, Otani S, Matsui-Yuasa I. Restricted Zn2+ availability affects the antizyme-dependent onrithine decarboxylase degradation pathway in isolated primary cultured rat hepatocytes. Biochem Biophys Res Commun. 1998. 243:797–800.16. Nakatini T, Tawaramoto M, Opare KD, Kojima A, Matsui-Yuasa I. Apoptosis induced by chelation of intracellular zinc is associated with depletion of cellular redubced glutathione level in rat hepatocytes. Chem Biol Interact. 2000. 125:151–163.17. Ovesen J, Moller-Madsen B, Thomsen JS, Danscher G, Mosekilde L. The positive effects of zinc on skeletal strength in growing rats. Bone. 2001. 29:565–570.
Article18. Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG. Zinc a potent inhibitor of the apoptotic protease caspase-3: a novel target for zinc in the inhibition of apoptosis. J Biol Chem. 1997. 272:18530–18533.19. Petrie L, Chesters JK, Franklin M. Inhibition of myoblast differentiation by lack of zinc. Biochem J. 1991. 276:109–111.
Article20. Shumaker DK, Vann LR, Goldberg MW, Allen TD, Wilson K. TPEN, a Zn2+/Fe2+ chelator with low affinity for Ca2+, inhibits lamin assembly, destabilizes nuclear architecture and may independently protect nuclei from apoptosis in vitro. Cell Calcium. 1998. 23:151–164.
Article21. Swanson JE, Feigenson GW. Thermodynamics of mixing of phosphatidylserine/phosphatidylcholine from measurements of high-affinity calcium binding. Biochemistry. 1990. 29:8291–8297.
Article22. Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomed Pharmacother. 2003. 57:399–411.
Article23. Telford W, Fraker P. Preferential induction of apoptosis in mouse CD4+CD8+TCR10CD310 thymocytes by zinc. J Cell Physiol. 1995. 164:259–270.24. Westin KJ, Rasmuson AC. Nucleation of calcium carbonate in presence of citric acid, DTPA, EDTA and pyromellitic acid. J Colloid Interface Sci. 2005. 282:370–379.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The function of zinc in the primary vascular smooth muscle cell proliferation in rats
- Cellular zinc deficiency inhibits the mineralized nodule formation and downregulates bone-specific gene expression in osteoblastic MC3T3-E1 cells
- Spironolactone Attenuates Methylglyoxal-induced Cellular Dysfunction in MC3T3-E1 Osteoblastic Cells
- Lactoferrin Constitutively Enhances Differentiation of Osteoblastic MC3T3-E1 Cells in Vitro
- New DNA Extraction Method for Diagnosis of Tuberculosis by Polymerase Chain Reaction