Lab Med Online.
2012 Jul;2(3):139-147.
Analysis of Polycheck Allergy Results of the Recent Two Years: Comparison with Skin Prick Test and ImmunoCAP
- Affiliations
-
- 1Department of Laboratory Medicine, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea. jhyoo92@empal.com
- 2Department of Pediatrics, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- 3Department of Dermatology, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Biochemistry and Cell Biology, Rice University, Houston, Texas, USA.
Abstract
- BACKGROUND
Multiple Antigen Simultaneous Test (MAST)-immunoblot assay is a practical and economical test, which has been recently introduced nationwide. Authors investigated test efficiency of a MAST-immunoblot assay, Polycheck Allergy (PA).
METHODS
A total of 3,153 patients were tested by PA and the results were compared with the results of ImmunoCAP and skin prick test (SPT) in 532 and 75 patients, respectively. The correlation with the lgE results measured by VIDAS was also analyzed.
RESULTS
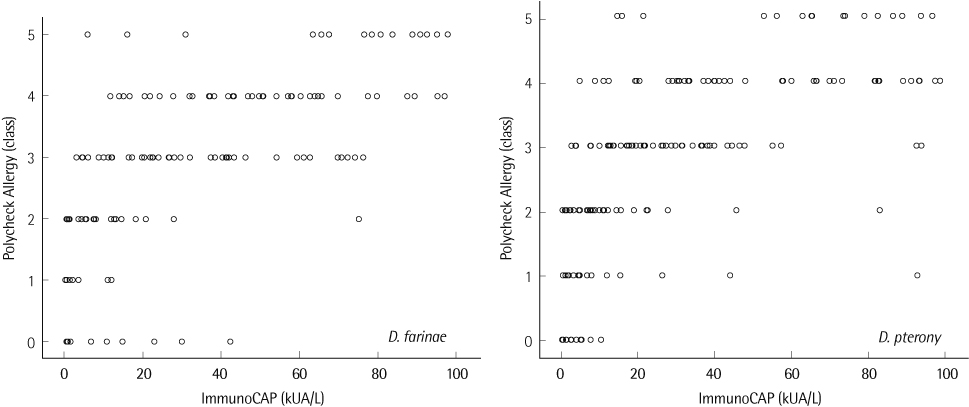
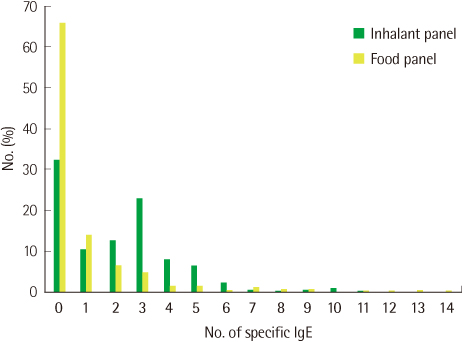
The agreements of PA with SPT were 87.8% in the Inhalant Panel and 89.3% in the Food Panel and the agreement of ImmunoCAP with SPT was 95.2%. The most common allergens giving positive reactions were Dermatophagoides farinae (46.2%) and Dermatophagoides pteronyssinus (40.0%). SPT taken as a reference, PA compared with ImmunoCAP showed higher agreement (D. farinae, 76.0 vs. 70.7%; D. pteronyssinus, 76.0 vs. 74.4%), sensitivity (D. farinae, 72.7 vs. 68.2%; D. pteronyssinus, 75.0 vs. 71.2%) and specificity (D. farinae, 85.0 vs. 81.3%) except for the specificity for D. pteronyssinus (78.3 vs. 87.5%). The rate of allergen specific IgE positive patients was higher than that of negative patients when total IgE was over 100 kU/L.
CONCLUSIONS
Our results showed that the agreement, sensitivity and specificity of PA were similar to or better than those of the previously established test, ImmunoCAP. The allergen specific IgE results of PA were in correlation with total IgE. PA may be used for testing allergen specific IgE to diagnose and treat allergic diseases.
MeSH Terms
Figure
Reference
-
1. Min YG, Jung HW, Kim HS, Park SK, Yoo KY. Prevalence and risk factors for perennial allergic rhinitis in Korea: results of a nationwide survey. Clin Otolaryngol Allied Sci. 1997. 22:139–144.
Article2. Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, et al. Prevalences of symptoms of asthma and other allergic diseases in korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001. 16:155–164.
Article3. Suh M, Kim HH, Sohn MH, Kim KE, Kim C, Shin DC. Prevalence of allergic diseases among Korean school-age children: a nationwide cross-sectional questionnaire study. J Korean Med Sci. 2011. 26:332–338.
Article4. Douglass JA, O'Hehir RE. 1. Diagnosis, treatment and prevention of allergic disease: the basics. Med J Aust. 2006. 185:228–233.
Article5. Nelson HS. Diagnostic procedures in allergy. I. Allergy skin testing. Ann Allergy. 1983. 51:411–418.6. Ownby DR. Allergy testing: in vivo versus in vitro. Pediatr Clin North Am. 1988. 35:995–1009.
Article7. Pipkorn U. Pharmacological influence of antiallergic medication on in vivo allergen testing. Allergy. 1988. 43:81–86.
Article8. Son BK, Lim DH. Allergic skin test. Korean J Pediatr. 2007. 50:409–415.
Article9. Wide L, Bennich H, Johansson SG. Diagnosis of allergy by an in-vitro test for allergen antibodies. Lancet. 1967. 2:1105–1107.
Article10. Lim HS, Kim HS, Oh H. Current status of serum allergen tests in Korea. Korean J Lab Med. 2008. 28:124–129.
Article11. Brown CR, Higgins KW, Frazer K, Schoelz LK, Dyminski JW, Marinkovich VA, et al. Simultaneous determination of total IgE and allergen-specific IgE in serum by the MAST chemiluminescent assay system. Clin Chem. 1985. 31:1500–1505.
Article12. Lim HS, Yoon JK, Lee HH. Allergen patterns using MAST CLA test in Korean pediatric patients. Korean J Clin Pathol. 2001. 21:292–297.13. Yang SE, Oh HB, Hong SJ, Moon DH, Chi HS. Analysis of MAST chemiluminescent assay (MAST CLA) results performed in Asan Medical Center: Suggestion for the improvement of MAST CLA performance. Korean J Clin Pathol. 1998. 18:660–666.14. Dreborg S. The skin prick test in the diagnosis of atopic allergy. J Am Acad Dermatol. 1989. 21:820–821.
Article15. Mosbech H, Nielsen NH, Dirksen A, Launbjerg J, Biering I, Søborg M. Comparison between specific IgE measured by RAST, two chemiluminescent assays and skin prick test. Allergol Immunopathol (Madr). 1992. 20:220–224.16. Eiken P, Mosbech H, Jacobsen L, Larsen P, Scharling B, Svendsen UG, et al. Comparison between two different assays for measurements of allergen-specific IgE and skin prick test in the diagnosis of insect venom allergy. Allergy. 1992. 47:495–497.
Article17. Scolozzi R, Boccafogli A, Vicentini L, Baraldi A, Bagni B. Correlation of MAST chemiluminescent assay (CLA) with RAST and skin prick tests for diagnosis of inhalant allergic disease. Ann Allergy. 1989. 62:193a–193b.18. Lee S, Lim HS, Park J, Kim HS. A new automated multiple allergen simultaneous test-chemiluminescent assay (MAST-CLA) using an AP720S analyzer. Clin Chim Acta. 2009. 402:182–188.
Article19. Jang WR, Nahm CH, Kim JH, Lim DH, Jang TY, Moon YS, et al. Allergen specific IgE measurement with Polycheck Allergy: comparison of three multiple allergen simultaneous tests. Korean J Lab Med. 2009. 29:465–472.
Article20. Contin-Bordes C, Petersen A, Chahine I, Boralevi F, Chahine H, Taïeb A, et al. Comparison of ADVIA Centaur and Pharmacia UniCAP tests in the diagnosis of food allergy in children with atopic dermatitis. Pediatr Allergy Immunol. 2007. 18:614–620.
Article21. Cha YJ, Chae SL, Park AJ. Evaluation of the MAST CLA assay system for measuring total IgE: Comparison with the pharmacia CAP system. Korean J Clin Pathol. 1999. 19:342–347.22. Nepper-Christensen S, Backer V, DuBuske LM, Nolte H. In vitro diagnostic evaluation of patients with inhalant allergies: summary of probability outcomes comparing results of CLA- and CAP-specific immunoglobulin E test systems. Allergy Asthma Proc. 2003. 24:253–258.23. Kim HS, Kim DJ, Lee SG. Analysis of simultaneous positivity to multiple allergens on MAST CLA Test. Korean J Lab Med. 2005. 25:448–456.24. Agha F, Sadaruddin A, Abbas S, Ali SM. Serum IgE levels in patients with allergic problems and healthy subjects. J Pak Med Assoc. 1997. 47:166–169.25. Backer V, Ulrik CS, Wendelboe D, Bach-Mortensen N, Hansen KK, Laursen EM, et al. Distribution of serum IgE in children and adolescents aged 7 to 16 years in Copenhagen, in relation to factors of importance. Allergy. 1992. 47:484–489.
Article26. Sears MR, Chow CM, Morseth DJ. Serum total IgE in normal subjects and the influence of a family history of allergy. Clin Allergy. 1980. 10:423–431.
Article27. Peat JK, Toelle BG, Dermand J, van den Berg R, Britton WJ, Woolcock AJ. Serum IgE levels, atopy, and asthma in young adults: results from a longitudinal cohort study. Allergy. 1996. 51:804–810.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allergen Specific IgE Measurement with Polycheck Allergy: Comparison of Three Multiple Allergen Simultaneous Tests
- Comparison of skin prick test and serum specific IgE measured by ImmunoCAP system for various inhalant allergens
- Bird-Egg syndrome diagnosed by ImmunoCap ISAC
- Comparative Study of ImmunoCAP and ELISA for Detecting Allergen Specific IgE to Japanese Hop Pollen and Two Spotted Spider Mite
- Comparison of Multiple Allergen Simultaneous Test and ImmunoCAP for the Diagnosis of Allergic Rhinitis