Korean J Urol.
2008 Jun;49(6):510-514.
Prognostic Significance of Multifocal Tumor in Radical Prostatectomy
- Affiliations
-
- 1Department of Urology, College of Medicine, Pochon CHA University, Korea.
- 2Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea. selee@snubh.org
- 3Department of Pathology, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Urology, Seoul National University Hospital, Seoul, Korea.
Abstract
-
PURPOSE: We investigate the impact of tumor multifocality on the biochemical recurrence rate after radical prostatectomy.
MATERIALS AND METHODS
Data was collected from 525 patients who underwent radical prostatectomy for clinically localized prostate cancer from 2003 to 2007. We evaluated the potential associations of multifocality with various clinical and pathologic factors. The ability to predict extra-capsular extension(ECE) was tested by logistic regression models, whereas biochemical recurrence(BCR) was assessed via Kaplan-Meier analyses and Cox-hazard regression models. The BCR was defined as a level of serum prostate-specific antigen(PSA) of 0.2ng/ml or greater on consecutive evaluations.
RESULTS
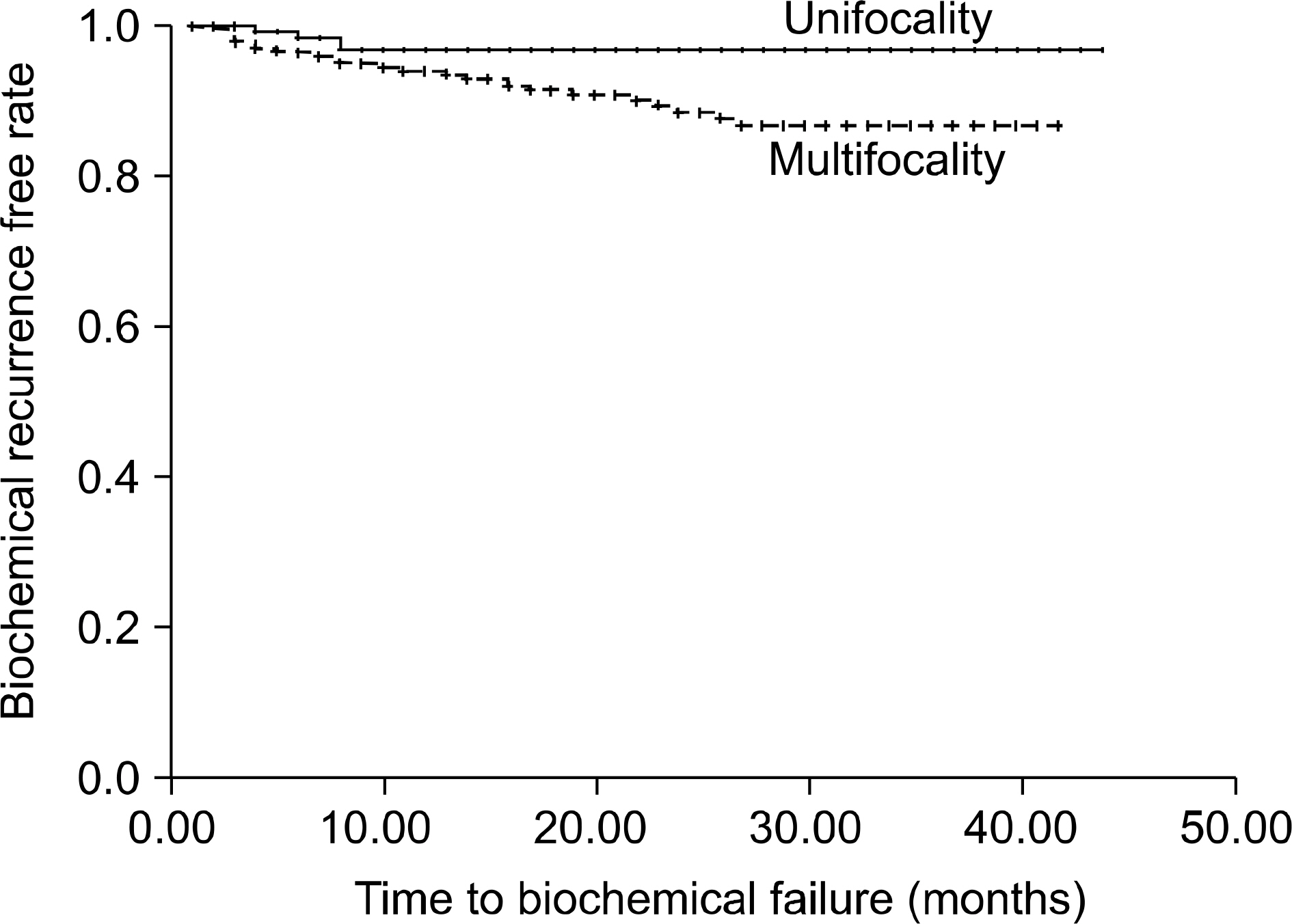
Multifocality was observed to be significantly associated with the presence of a high grade Gleason pattern(p=0.014), the pT stage(p< 0.001), ECE(p=0.005) and a positive surgical margin(PSM)(p=0.019). Moreover, it was the independent predictor of ECE on multivariate logistic regression analyses(p=0.039). However, although multifocality had a significant influence on biochemical recurrence on the Kaplan-Meier analyses (log rank test, p=0.019), only the PSA level and the Gleason score were significant predictors of BCR on the multivariated Cox-hazard analyses.
CONCLUSIONS
Although multifocality was associated with adverse pathologic features, it had no significant effect on biochemical recurrence on the multivariated cox-hazard analyses.
Keyword
Figure
Reference
-
1. Smither AR, Guralnick ML, Davis NB, See WA. Quantifying the natural history of post-radical prostatectomy incontinence using objective pad test data. BMC Urol. 2007; 7:2.
Article2. Matthew AG, Goldman A, Trachtenberg J, Robinson J, Horsburgh S, Currie K, et al. Sexual dysfunction after radical prostatectomy: prevalence, treatments, restricted use of treatments and distress. J Urol. 2005; 174:2105–10.
Article3. Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002; 167:1231–4.
Article4. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994; 271:368–74.
Article5. Rakovitch E, Pignol JP, Hanna W, Narod S, Spayne J, Nofech-Mozes S, et al. Significance of multifocality in ductal carcinoma in situ: outcomes of women treated with breast-conserving therapy. J Clin Oncol. 2007; 25:5591–6.
Article6. Santiago RJ, Wu L, Harris E, Fox K, Schultz D, Glick J, et al. Fifteen-year results of breast-conserving surgery and definitive irradiation for stage I and II breast carcinoma: the University of Pennsylvania experience. Int J Radiat Oncol Biol Phys. 2005; 58:233–40.
Article7. Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol. 2003; 169:517–23.
Article8. Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997; 277:1445–51.
Article9. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998; 90:766–71.
Article10. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002; 167:528–34.
Article11. Chun FK, Briganti A, Jeldres C, Gallina A, Erbersdobler A, Schlomm T, et al. Tumour volume and high grade tumour volume are the best predictors of pathologic stage and biochemical recurrence after radical prostatectomy. Eur J Cancer. 2007; 43:536–43.
Article12. Ates M, Teber D, Gozen AS, Tefekli A, Sugiono M, Hruza M, et al. Do tumor volume, tumor volume ratio, type of nerve sparing and surgical experience affect prostate specific antigen recurrence after laparoscopic radical prostatectomy? A matched pair analysis. J Urol. 2007; 177:1771–5.
Article13. McNeal JE, Price HM, Redwine EA, Freiha FS, Stamey TA. Stage A versus stage B adenocarcinoma of the prostate: morphological comparison and biological significance. J Urol. 1988; 139:61–5.
Article14. Villers A, McNeal JE, Freiha FS, Stamey TA. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992; 70:2313–8.
Article15. Miller GJ, Cygan JM. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994; 152:1709–13.
Article16. Djavan B, Susani M, Bursa B, Basharkhah A, Simak R, Marberger M. Predictability and significance of multifocal prostate cancer in the radical prostatectomy specimen. Tech Urol. 1999; 5:139–42.17. Van Oort IM, Schout BM, Kiemeney LA, Hulsbergen CA, Witjes JA. Does the tertiary Gleason pattern influence the PSA progression-free interval after retropubic radical prostatectomy for organ-confined prostate cancer? Eur Urol. 2005; 48:572–6.
Article18. Pan CC, Potter SR, Partin AW, Epstein JI. The prognostic significance of tertiary Gleason patterns of higher grade in radical prostatectomy specimens: a proposal to modify the Gleason grading system. Am J Surg Pathol. 2000; 24:563–9.19. Ruijter ET, van De Kaa CA, Schalken JA, Debruyne FM, Ruiter DJ. Histological grade heterogeneity in multifocal prostate cancer. Biological and clinical implications. J Pathol. 1996; 180:295–9.
Article20. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994; 271:368–74.
Article21. Eichelberger LE, Koch MO, Daggy JK, Ulbright TM, Eble JN, Cheng L. Predicting tumor volume in radical prostatectomy specimens from patients with prostate cancer. Am J Clin Pathol. 2003; 120:386–91.
Article22. Noguchi M, Stamey TA, McNeal JE, Nolley R. Prognostic factors for multifocal prostate cancer in radical prostatectomy specimens: lack of significance of secondary cancers. J Urol. 2003; 170:459–63.
Article23. Eggener SE, Scardino PT, Carroll PR, Zelefsky MJ, Sartor O, Hricak H, et al. Focal therapy for localized prostate cancer: a critical appraisal of rationale and modalities. J Urol. 2007; 178:2260–7.
Article24. Gburek BM, Kollmorgen TA, Qian J, D'Souza-Gburek SM, Lieber MM, Jenkins RB. Chromosomal anomalies in stage D1 prostate adenocarcinoma primary tumors and lymph node metastases detected by fluorescence in situ hybridization. J Urol. 1997; 157:223–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Clinical and Pathological Findings in Unifocal and Multifocal Prostate Cancer
- The Prognostic Significance of Neuroendocrine Differentiation for Treating Prostatic Carcinoma in 699 Cases of Radical Prostatectomy
- Radical Prostatectomy
- Clinicopathological Significance of the Lymphovascular Invasion Detected in Specimens from Radical Retropubic Prostatectomies
- A Case of No Residual Cancer in Radical Prostatectomy Specimens Despite Biopsy-proven Prostate Cancer