Korean J Urol.
2009 Apr;50(4):320-326.
Analysis of the Clinicopathologic Characteristics of Men with Prostate Cancer Undergoing Radical Prostatectomy in the Prostate-Specific Antigen Range of Less than 4 ng/ml
- Affiliations
-
- 1Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. cskim@amc.seoul.kr
Abstract
- PURPOSE
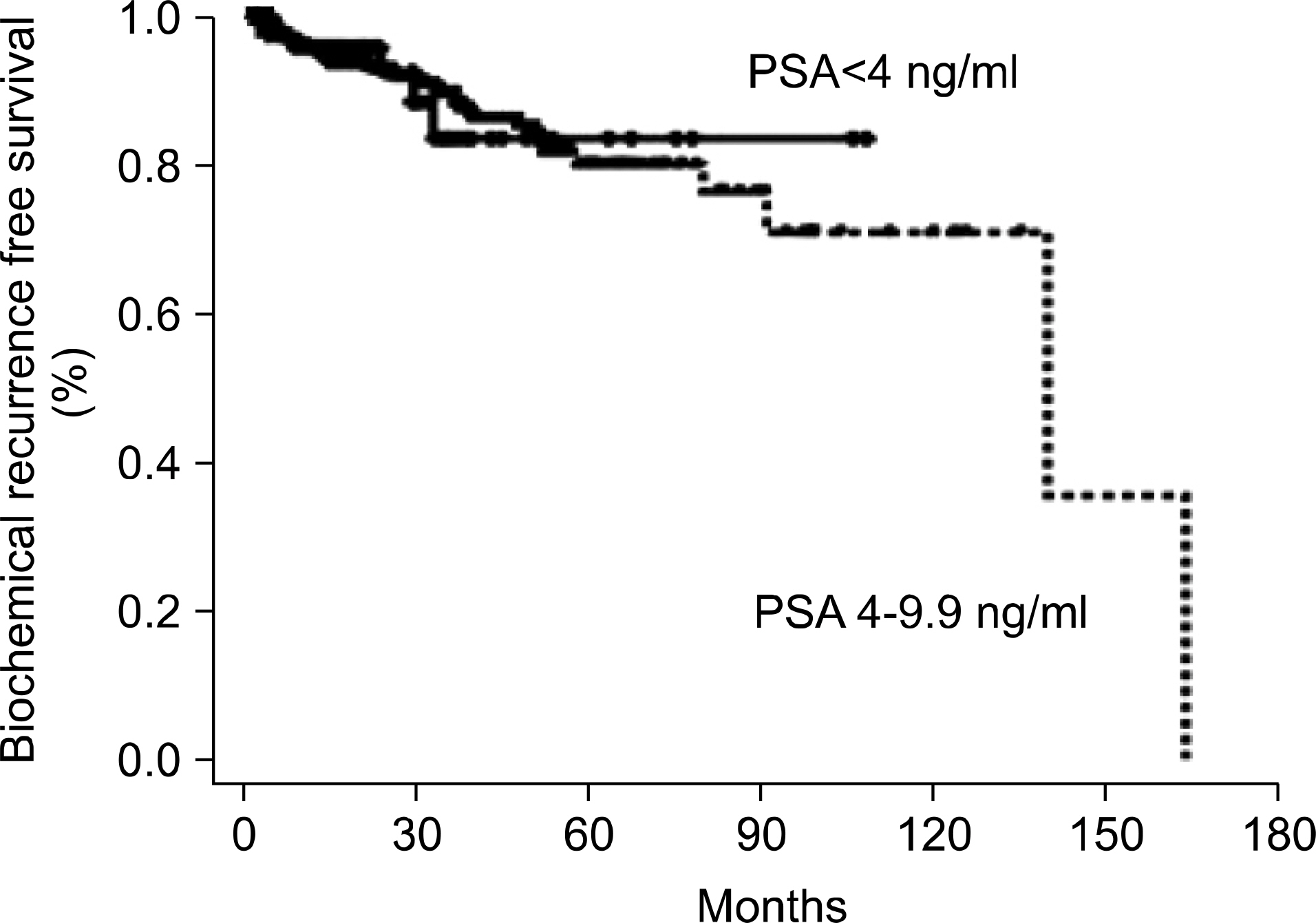
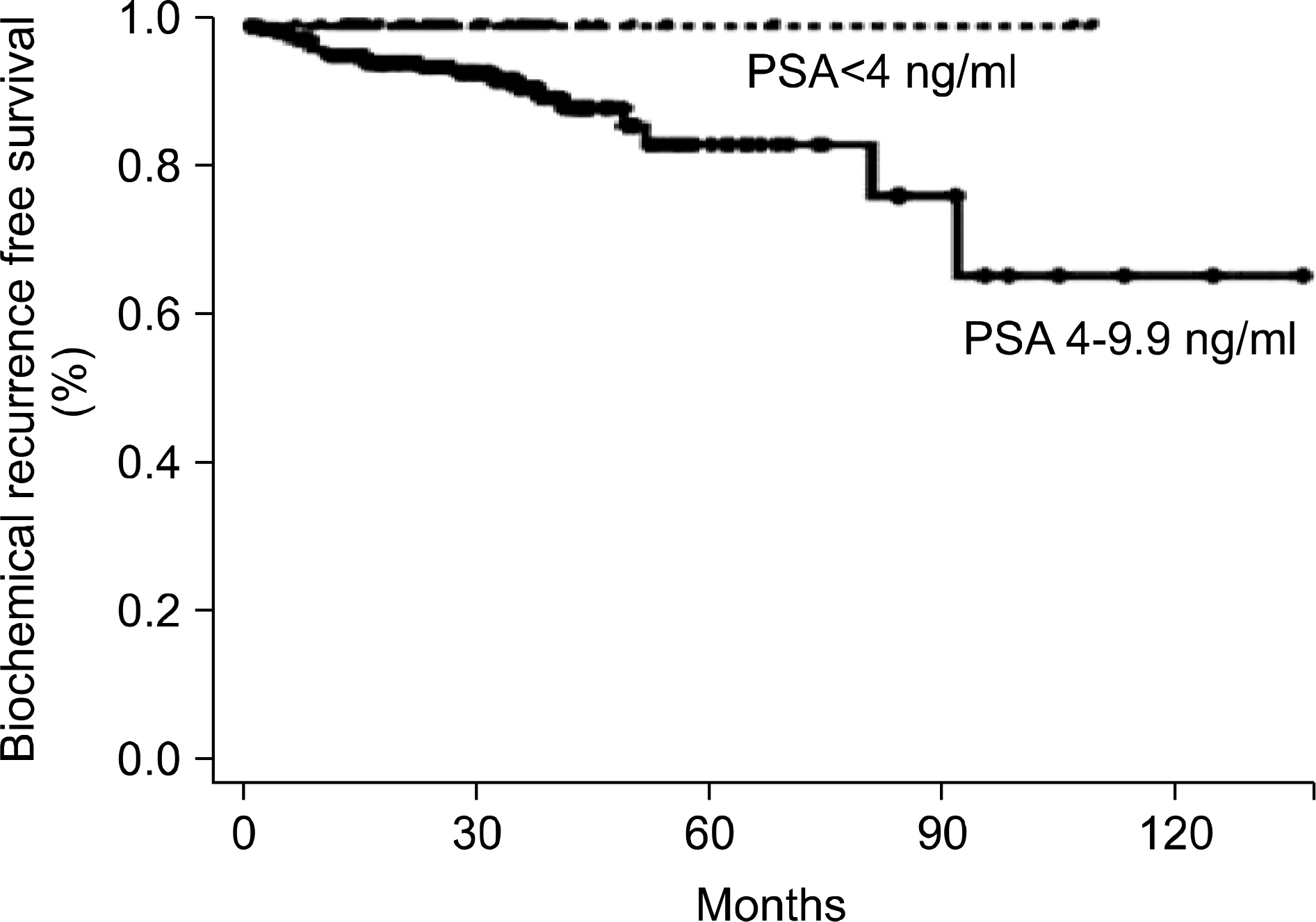
We analyzed the clinicopathologic characteristics of men undergoing radical prostatectomy in the prostate-specific antigen (PSA) range of less than 4 ng/ml and compared this with the results for men who had a PSA range of 4 to 9.9 ng/ml. MATERIALS AND METHODS: The study population consisted of 447 men treated for prostate cancer with radical prostatectomy between 1990 and 2006 at our institute who had a prebiopsy PSA of less than 10 ng/ml. The average follow-up period was 37 months. Clinicopathologic characteristics were compared between men with a PSA value of less than 4 ng/ml (low-PSA group) and men with a value in the range of 4 to 9.9 ng/ml (intermediate-PSA group). Survival analysis was performed by the Kaplan-Meier method and Cox proportional hazard regression analysis. RESULTS: Of these 447 patients, 60 (13.4%) and 387 (86.6%) had a low or an intermediate prebiopsy PSA level, respectively. The pathologic findings of the prostatectomy specimens showed no significant differences between the 2 groups, including Gleason score and pathologic stage. The 5-year biochemical recurrence-free survival in the low- and intermediate-PSA groups was 82.8% and 79.3%, respectively, and there was no significant difference between the 2 groups (p=0.946). Multivariate analysis showed that, in the entire cohort, pathologic Gleason score and lymph node involvement were independent predictors of biochemical recurrence. CONCLUSIONS: No statistically significant differences were found in clinicopathologic characteristics or clinical outcome between the low- and intermediate-PSA groups. These results suggest that a lower PSA cutoff should be considered as an indication for prostate biopsy in the Korean population.
MeSH Terms
Figure
Reference
-
1.Catalona WJ., Smith DS., Ratliff TL., Dodds KM., Coplen DE., Yuan JJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991. 324:1156–61.
Article2.Cooner WH., Mosley BR., Rutherford CL Jr., Beard JH., Pond HS., Bass RB Jr, et al. Clinical application of transrectal ultrasonography and prostate specific antigen in the search for prostate cancer. J Urol. 1988. 139:758–61.
Article3.Thompson IM., Ankerst DP., Chi C., Lucia MS., Goodman PJ., Crowley JJ, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005. 294:66–70.
Article4.Oesterling JE., Jacobsen SJ., Chute CG., Guess HA., Girman CJ., Panser LA, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993. 270:860–4.
Article5.Catalona WJ., Ramos CG., Carvalhal GF., Yan Y. Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology. 2000. 55:791–5.
Article6.de Koning HJ., Auvinen A., Berenguer Sanchez A., Calais da Silva F., Ciatto S., Denis L, et al. Large-scale randomized prostate cancer screening trials: program performances in the European Randomized Screening for Prostate Cancer trial and the Prostate, Lung, Colorectal and Ovary cancer trial. Int J Cancer. 2002. 97:237–44.
Article7.Postma R., Schroder FH. Screening for prostate cancer. Eur J Cancer. 2005. 41:825–33.
Article8.Vis AN., Kranse R., Roobol M., van der Kwast TH., Schroder FH. Serendipity in detecting disease in low prostate-specific antigen ranges. BJU Int. 2002. 89:384–9.
Article9.Ku JH. Race-specific reference ranges of serum prostate-specific antigen levels in countries with a low incidence of prostate cancer. BJU Int. 2006. 97:69–72.
Article10.Park HK., Hong SK., Byun SS., Lee SE. T1c prostate cancer detection rate and pathologic characteristics: comparison between patients with serum prostate-specific antigen range of 3.0 to 4.0 ng/mL and 4.1 to 10.0 ng/mL in Korean population. Urology. 2006. 68:85–8.
Article11.Hankey BF., Feuer EJ., Clegg LX., Hayes RB., Legler JM., Prorok PC, et al. Cancer surveillance series: interpreting trends in prostate cancer—part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999. 91:1017–24.
Article12.Aus G., Abbou CC., Bolla M., Heidenreich A., Schmid HP., van Poppel H, et al. EAU guidelines on prostate cancer. Eur Urol. 2005. 48:546–51.
Article13.Shekarriz B., Upadhyay J., Bianco FJ Jr., Tefilli MV., Tiguert R., Gheiler EL, et al. Impact of preoperative serum PSA level from 0 to 10 ng/ml on pathological findings and disease-free survival after radical prostatectomy. Prostate. 2001. 48:136–43.
Article14.Aleman M., Karakiewicz PI., Kupelian P., Kattan MW., Graefen M., Cagiannos I, et al. Age and PSA predict likelihood of organ-confined disease in men presenting with PSA less than 10 ng/ml: implications for screening. Urology. 2003. 62:70–4.
Article15.Berger AP., Volgger H., Rogatsch H., Strohmeyer D., Steiner H., Klocker H, et al. Screening with low PSA cutoff values results in low rates of positive surgical margins in radical prostatectomy specimens. Prostate. 2002. 53:241–5.
Article16.Thompson IM., Pauler DK., Goodman PJ., Tangen CM., Lucia MS., Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004. 350:2239–46.17.Catalona WJ., Smith DS., Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/ml and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997. 277:1452–5.
Article18.Gann PH., Hennekens CH., Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995. 273:289–94.
Article19.Lee HW., Kwak WK., Choi YH., Choi HY., Lee HM. New thresholds for prostate-specific antigen velocity for prostate cancer screening in Korean patients younger than 60 years old. Korean J Urol. 2008. 49:113–7.
Article20.Chung JS., Han BK., Jeong SJ., Hong SK., Byun SS., Choe G, et al. Prognostic significance of the tumor volume and tumor percentage for localized prostate cancer. Korean J Urol. 2008. 49:1074–80.
Article21.Stamey TA., Johnstone IM., McNeal JE., Lu AY., Yemoto CM. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002. 167:103–11.
Article22.Welch HG., Schwartz LM., Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst. 2005. 97:1132–7.
Article23.Freedland SJ., Aronson WJ., Kane CJ., Terris MK., Presti JC Jr., Trock B, et al. Biochemical outcome after radical prostatectomy among men with normal preoperative serum prostate-specific antigen levels. Cancer. 2004. 101:748–53.
Article24.Antenor JA., Roehl KA., Eggener SE., Kundu SD., Han M., Catalona WJ. Preoperative PSA and progression-free survival after radical prostatectomy for Stage T1c disease. Urology. 2005. 66:156–60.
Article25.Schmid HP., Riesen W., Prikler L. Update on screening for prostate cancer with prostate-specific antigen. Crit Rev Oncol Hematol. 2004. 50:71–8.
Article26.Yang WJ., Lee DH., Chung BH., Cho JS., Choi YD., Kim SJ, et al. Detection rate of prostate cancer on biopsy according to serum prostate-specific antigen in Korean men: a multicenter study. Urology. 2006. 67:333–6.
Article27.Crawford ED., Thompson IM. Controversies regarding screening for prostate cancer. BJU Int. 2007. 100(Suppl 2):5–7.
Article28.Cho JS., Kim SI., Kim SJ., Kim YS., Kim CI., Kim HS, et al. Lowering prostate-specific antigen threshold for prostate biopsy in Korean men: impact on the number needing biopsy. Korean J Urol. 2008. 49:118–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radical Prostatectomy
- The Impact of PSA on Pathologic Characteristics in the Radical Prostatectomy with PSA Level of 4-10ng/ml
- Outcome of Radical Prostatectomy in Prostate Cancer Patients with Prostate-Specific Antigen (PSA) Level Equal to or More Than 20 ng/ml and No Distant Metastasis Preoperatively
- Association of Prostate Specific Antigen (PSA) Velocity with Age and Initial PSA in Healthy Korean Men
- pT3 Predictive Factors in Patients with a Gleason Score of 6 in Prostate Biopsies