J Dent Anesth Pain Med.
2015 Sep;15(3):121-128.
Hemodynamic changes associated with a novel concentration of lidocaine HCl for impacted lower third molar surgery
- Affiliations
-
- 1Faculty of Odonto-Stomatology, University of Health Sciences, Phnom Penh, Cambodia.
- 2Department of Oral Maxillofacial Surgery, Faculty of Dentistry, Mahidol University, Bangkok, Thailand. natthamet.won@mahidol.ac.th
- 3Department of Dentistry, University of Puthisastra, Phnom Penh, Cambodia.
- 4Dean of Faculty of Odonto-Stomatology, University of Health Sciences, Phnom Penh, Cambodia.
- 5Research office, Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
Abstract
- BACKGROUND
The authors studied the hemodynamic effect influent by using the novel high concentration of lidocaine HCl for surgical removal impacted lower third molar. The objective of this study was to evaluate the hemodynamic change when using different concentrations of lidocaine in impacted lower third molar surgery.
METHODS
Split mouth single blind study comprising 31 healthy patients with a mean age of 23 years (range 19-33 years). Subjects had symmetrically impacted lower third molars as observed on panoramic radiograph. Each participant required 2 surgical interventions by the same surgeon with a 3-week washout period washout period. The participants were alternately assigned one of two types of local anesthetic (left or right) for the first surgery, then the other type of anesthetic for the second surgery. One solution was 4% lidocaine with 1:100,000 epinephrine and the other was 2% lidocaine with 1:100,000 epinephrine. A standard IANB with 1.8 ml volume was used. Any requirement for additional anesthetic and patient pain intra-operation was recorded. Post-operatively, patient was instructed to fill in the patient report form for any adverse effect and local anesthetic preference in terms of intra-operative pain. This form was collected at the seven day follow up appointment.
RESULTS
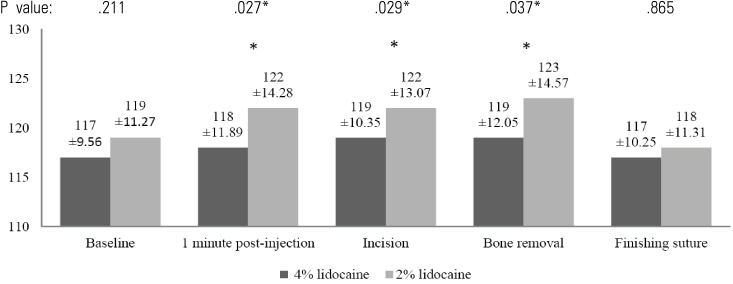
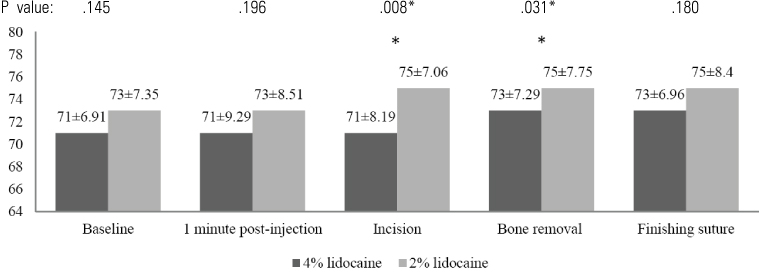
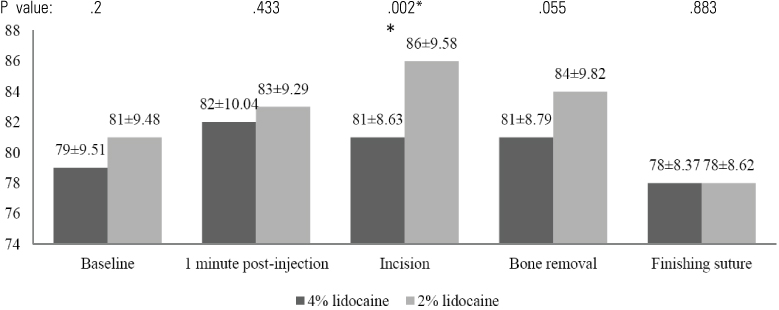
In the 4% lidocaine group, the heart rate increased during the first minute post-injection (P < 0.05). However, there was no significant change in arterial blood pressure during the operation. In the 2% lidocaine group, there was a significant increase in arterial blood pressure and heart rate in the first minute following injection for every procedure. When the hemodynamic changes in each group were compared, the 4% lidocaine group had significantly lower arterial blood pressure compared to the 2% lidocaine group following injection. Post-operatively, no adverse effects were observed by the operator and patient in either local anesthetic group. Patients reported less pain intra-operation in the 4% lidocaine group compared with the 2% lidocaine group (P < .05).
CONCLUSIONS
Our results suggest that a 4% concentration of lidocaine HCl with 1:100,000 epinephrine has better clinical efficacy than 2% lidocaine HCl with 1:100,000 epinephrine when used for surgical extraction of lower third molars. Neither drug had any clinical adverse effects.
Keyword
MeSH Terms
Figure
Reference
-
1. McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiol. 2005; 71:59–74.2. Malamed SF. Handbook of Local Anesthesia. Sixth edition. 2014.3. Ping B, Kiattavorncharoen S, Saengsirinavin C, Im P, Durward CS. Effect of high concentration lidocaine for mandibular teeth anesthesia: Review of literature. M Dent J. 2014; 34:364–372.4. Brand HS, Abraham-Inpijn L. Cardiovascular responses induced by dental treatment. Eur J Oral Sci. 1996; 104:245–252.
Article5. Sapira JD, Bron K. Human epinephrine secretion. Direct measurement of the secretion of epinephrine from the human adrenal medulla. J Clin Endocrinol Metab. 1971; 33:436–447.
Article6. Lipp M, Dick W, Daublander M, Fuder H, Stanton-Hicks M. Exogenous and endogenous plasma levels of epinephrine during dental treatment under local anesthesia. Reg Anesth. 1993; 18:6–12.7. Troullos ES, Goldstein DS, Hargreaves KM, Dionne RA. Plasma epinephrine levels and cardiovascular response to high administered doses of epinephrine contained in local anesthesia. Anesth Prog. 1987; 34:10–13.8. Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012; 59:90–101.
Article9. de Morais HH, de Santana Santos T, Araujo FA, Vajgel A, de Holanda Vasconcellos RJ. Hemodynamic changes comparing lidocaine HCl with epinephrine and articaine HCl with epinephrine. J Craniofac Surg. 2012; 23:1703–1708.
Article10. Malamed SF. Pain and anxiety in dentistry. Sedation: a guide to patient management. 4th ed. St Louis: Mosby;2003. p. 2–6.11. Ezmek B, Arslan A, Delilbasi C, Sencift K. Comparison of hemodynamic effects of lidocaine, prilocaine and mepivacaine solutions without vasoconstrictor in hypertensive patients. J Appl Oral Sci. 2010; 18:354.
Article12. Yagiela JA. Local anesthetics. Anesth Prog. 1991; 38:128–141.
Article13. Grant DA, Lie T, Clark SM, Adams DF. Pain and discomfort levels in patients during root surface debridement with sonic metal or plastic inserts. J Periodontol. 1993; 64:645–650.
Article14. Dionne RA, Goldstein DS, Wirdzek PR. Effects of diazepam premedication and epinephrine-containing local anesthetic on cardiovascular and plasma catecholamine responses to oral surgery. Anesth Analg. 1984; 63:640–646.
Article15. Santos CF, Modena KC, Giglio FP, et al. Epinephrine concentration (1:100,000 or 1:200,000) does not affect the clinical efficacy of 4% articaine for lower third molar removal: a double-blind, randomized, crossover study. J Oral Maxillofac Surg. 2007; 65:2445–2452.
Article16. Moore PA, Boynes SG, Hersh EV, et al. The anesthetic efficacy of 4 percent articaine 1:200,000 epinephrine: two controlled clinical trials. J Am Dent Assoc. 2006; 137:1572–1581.17. Malamed SF, Gagnon S, Leblanc D. Articaine hydrochloride: a study of the safety of a new amide local anesthetic. J Am Dent Assoc. 2001; 132:177–185.
Article18. Vreeland DL, Reader A, Beck M, Meyers W, Weaver J. An evaluation of volumes and concentrations of lidocaine in human inferior alveolar nerve block. J Endod. 1989; 15:6–12.
Article19. Rood JP. Inferior alveolar nerve blocks. The use of 5 per cent lignocaine. Br Dent J. 1976; 140:413–414.
Article20. Rood JP, Cannell H. Plasma levels of lignocaine after peri-oral injections of two different concentrations. Pharmacol Ther Dent. 1978; 3:45–47.21. Sandy J, Rood JP. Five per cent lignocaine solution in children\'s dentistry. J Dent. 1980; 8:312–314.
Article22. Eldridge DJ, Rood JP. A double-blind trial of 5 per cent lignocaine solution. Br Dent J. 1977; 142:129–130.
Article23. Lima JL Jr, Dias-Ribeiro E, Ferreira-Rocha J, et al. Comparison of buccal infiltration of 4% articaine with 1 : 100,000 and 1 : 200,000 epinephrine for extraction of maxillary third molars with pericoronitis: a pilot study. Anesth Prog. 2013; 60:42–45.
Article24. Hiller A, Karjalainen K, Balk M, Rosenberg PH. Transient neurological symptoms after spinal anaesthesia with hyperbaric 5% lidocaine or general anaesthesia. Br J Anaesth. 1999; 82:575–579.
Article25. Kanai Y, Katsuki H, Takasaki M. Lidocaine disrupts axonal membrane of rat sciatic nerve in vitro. Anesth Analg. 2000; 91:944–948.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The efficacy of an elevated concentration of lidocaine HCl in impacted lower third molar surgery
- Comparative Study for the Anesthetic Efficacy between Articaine HCl and Lidocaine HCl during the Surgical Extraction of Bilateral Mandibular Impacted Third Molars
- The anesthetic efficiency of retromolar infiltrations with two local anesthetic solutions of the same concentration in lower third molar surgery
- Comparison of lidocaine with articaine buccal injection in reducing complications following impacted mandibular third molar surgery: a split-mouth randomized clinical trial
- 4% lidocaine versus 4% articaine for inferior alveolar nerve block in impacted lower third molar surgery