Anat Cell Biol.
2016 Jun;49(2):116-124. 10.5115/acb.2016.49.2.116.
Perineal raphe with special reference to its extension to the anus: a histological study using human fetuses
- Affiliations
-
- 1Department of Anatomy, Histology and Embryology, Yanbian University Medical College, Yanji, China. zwjin@ybu.edu.cn
- 2Division of Internal Medicine, Iwamizawa Kojinkai Hospital, Iwamizawa, Japan.
- 3Department of Anatomy and Human Embryology, Institute of Embryology, Faculty of Medicine, Complutense University, Madrid, Spain.
- 4Department of Anatomy, School of Medicine, Georg-August-Universität Göttingen, Göttingen, Germany.
- KMID: 2308918
- DOI: http://doi.org/10.5115/acb.2016.49.2.116
Abstract
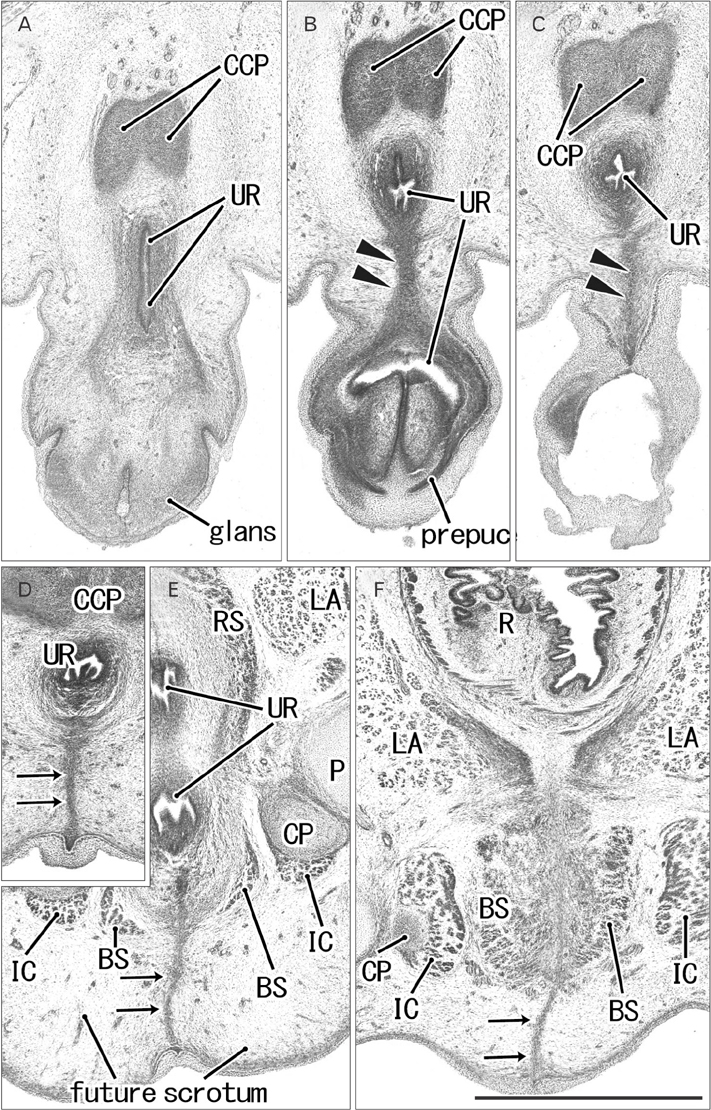
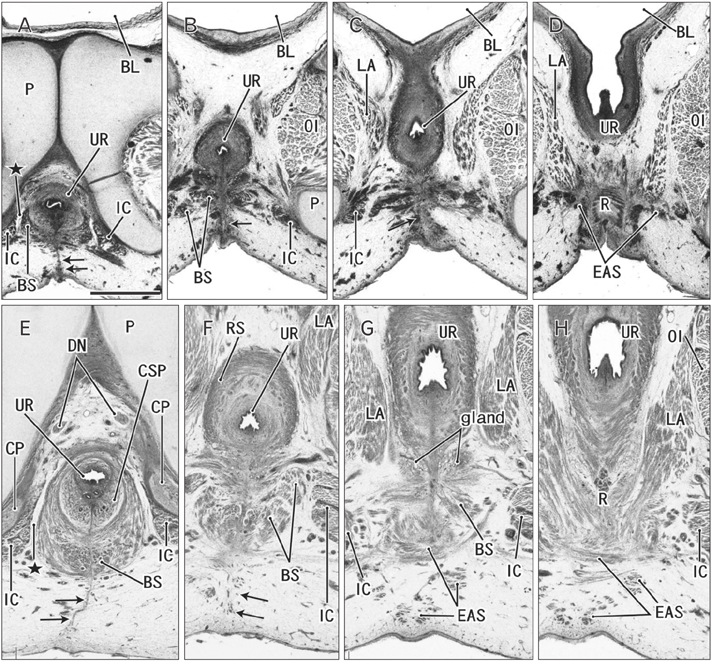
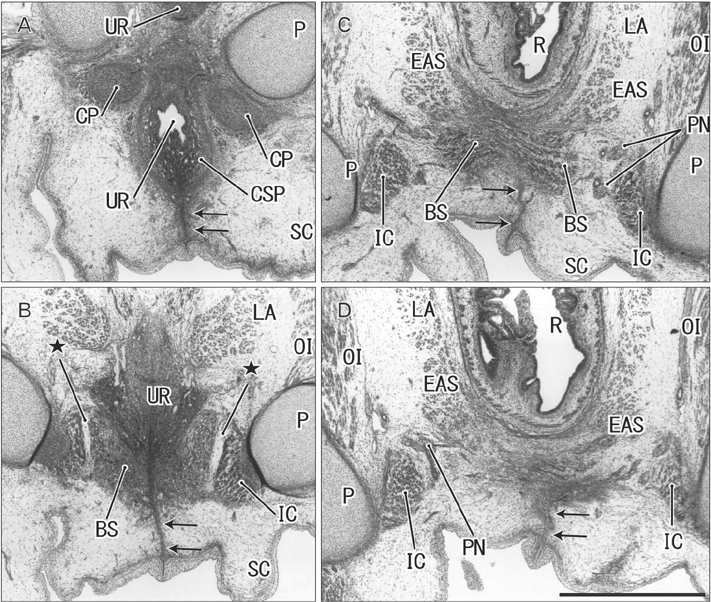
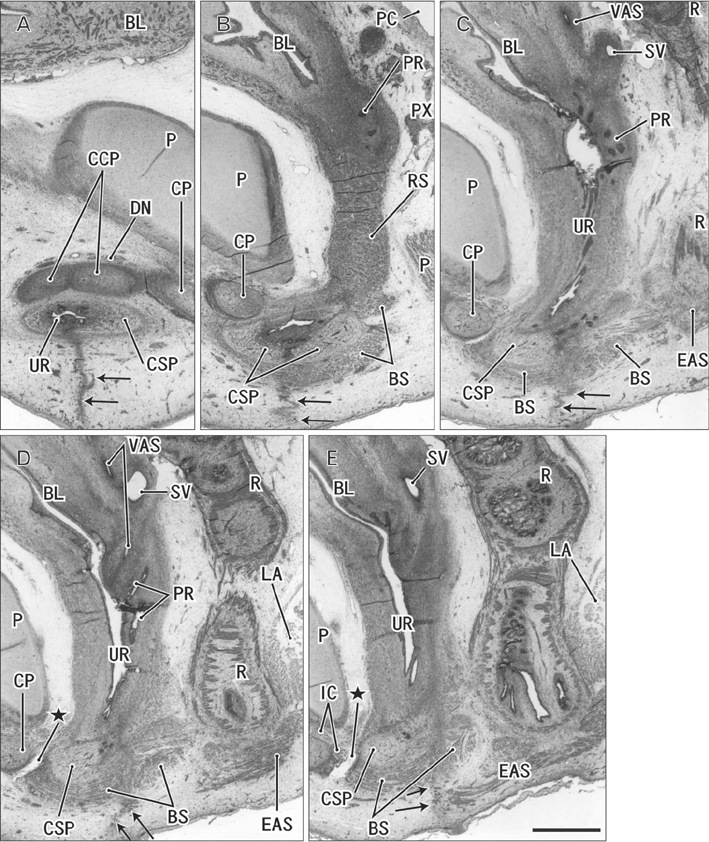
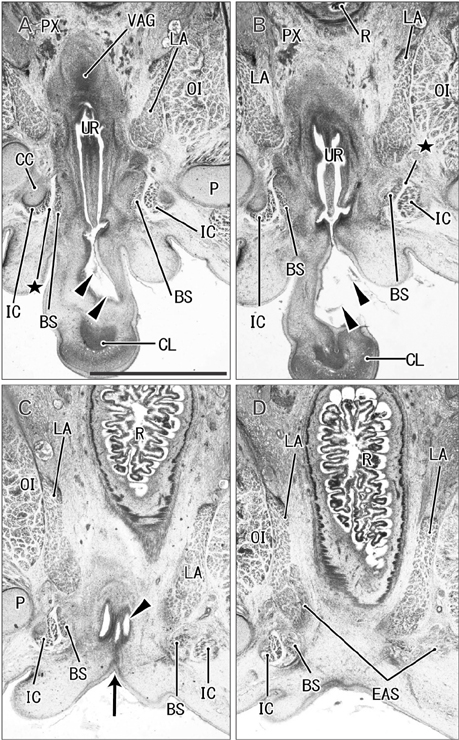
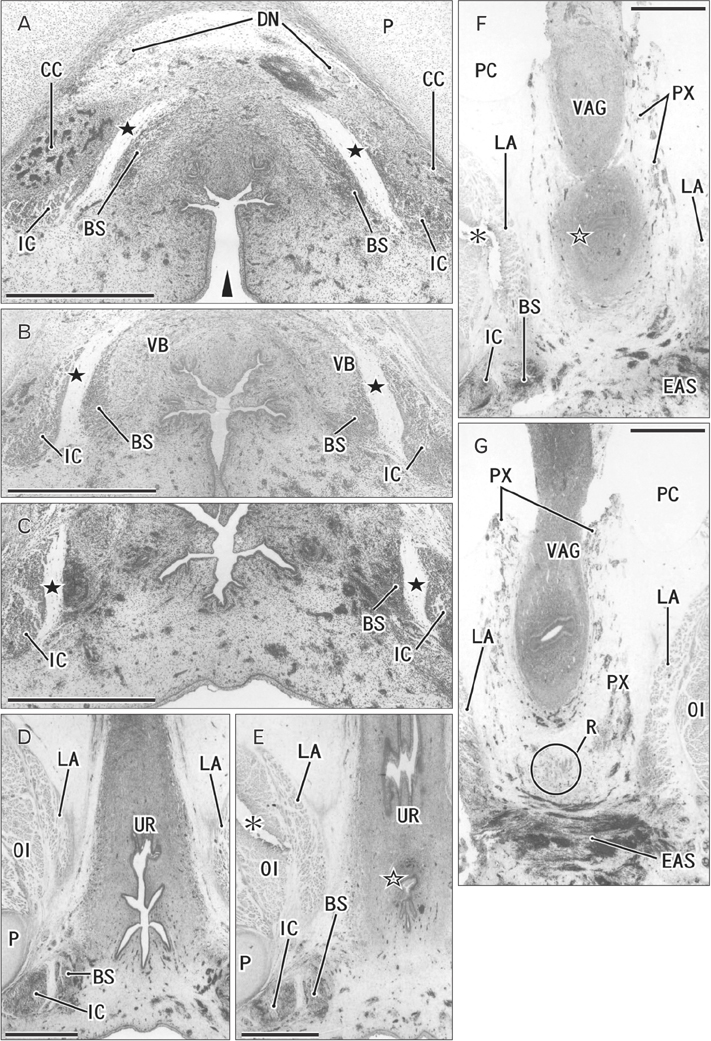
- The raphe of the human penis and scrotum is considered to develop secondarily after disappearance of the initial midline seam by fusion of the bilateral genital folds. However, the fetal development was still obscure. We examined histological sections of 30 fetuses (17 males and 13 females) at 10-15 weeks. In male fetuses, the scrotum was not yet clearly identified because of no descent of testis. The perineal raphe was thin and wavy at 10 weeks, and it was continuous with and took a direction same as the inferior wall of the closed penile urethra after physiological hypospadias. Depending on growth of the bulbospongiosus muscle and corpus spongiosus penis, the midline intermuscular septum obtained a connection to the subcutaneous wavy raphe and made the latter thick and straight at 12-15 weeks. Notably, the perineal raphe extended posteriorly to attach to the external anal sphincter. In female fetuses, an epithelial fusion occurred along a short distance at the posterior end of the vestibule. However, in front of the external anal sphincter, a large midline mesenchymal tissue from the urorectal septum did not contain a raphe-like structure. Moreover, since the bilateral bulbospongiosus muscles were separated widely by the vestibule, they did not provide a midline septum. Fetal development of the perineal raphe was accelerated by reinforcement from the muscular septum. In contrast, without such a muscular support, the female raphe could not maintain its growth even if the seed appeared at the posterior end of the vestibule.
MeSH Terms
Figure
Reference
-
1. Seifert AW, Harfe BD, Cohn MJ. Cell lineage analysis demonstrates an endodermal origin of the distal urethra and perineum. Dev Biol. 2008; 318:143–152.2. Georgas KM, Armstrong J, Keast JR, Larkins CE, McHugh KM, Southard-Smith EM, Cohn MJ, Batourina E, Dan H, Schneider K, Buehler DP, Wiese CB, Brennan J, Davies JA, Harding SD, Baldock RA, Little MH, Vezina CM, Mendelsohn C. An illustrated anatomical ontology of the developing mouse lower urogenital tract. Development. 2015; 142:1893–1908.3. Baskin LS, Erol A, Jegatheesan P, Li Y, Liu W, Cunha GR. Urethral seam formation and hypospadias. Cell Tissue Res. 2001; 305:379–387.4. Kim KS, Liu W, Cunha GR, Russell DW, Huang H, Shapiro E, Baskin LS. Expression of the androgen receptor and 5 alphareductase type 2 in the developing human fetal penis and urethra. Cell Tissue Res. 2002; 307:145–153.5. Niikura H, Okamoto S, Nagase S, Takano T, Murakami G, Tatsumi H, Yaegashi N. Fetal development of the human gubernaculum with special reference to the fasciae and muscles around it. Clin Anat. 2008; 21:547–557.6. de Vries PA, Friedland GW. The staged sequential development of the anus and rectum in human embryos and fetuses. J Pediatr Surg. 1974; 9:755–769.7. Kluth D, Hillen M, Lambrecht W. The principles of normal and abnormal hindgut development. J Pediatr Surg. 1995; 30:1143–1147.8. Nievelstein RA, van der Werff JF, Verbeek FJ, Valk J, Vermeij-Keers C. Normal and abnormal embryonic development of the anorectum in human embryos. Teratology. 1998; 57:70–78.9. Yamaguchi K, Kiyokawa J, Akita K. Developmental processes and ectodermal contribution to the anal canal in mice. Ann Anat. 2008; 190:119–128.10. van der Putte SC. The development of the human anorectum. Anat Rec (Hoboken). 2009; 292:951–954.11. Zhang T, Zhang HL, Wang DJ, Tang XB, Jia HM, Bai YZ, Yuan ZW, Wang WL. Normal development of hindgut and anorectum in human embryo. Int J Colorectal Dis. 2011; 26:109–116.12. van der Putte SC. The devlopment of the perineum in the human. A comprehensive histological study with a special reference to the role of the stromal components. Adv Anat Embryol Cell Biol. 2005; 177:1–131.13. Baky Fahmy MA. The spectrum of genital median raphe anomalies among infants undergoing ritual circumcision. J Pediatr Urol. 2013; 9(6 Pt A):872–877.14. Abdel Aleem A, el Sheikh S, Mokhtar A, Ghafouri H, Saleem M. The perineal groove and canal in males and females: a third look. Z Kinderchir. 1985; 40:303–307.15. Chatterjee SK, Chatterjee US, Chatterjee U. Perineal groove with penoscrotal hypospadias. Pediatr Surg Int. 2003; 19:554–556.16. Mullassery D, Turnock R, Kokai G. Perineal groove. J Pediatr Surg. 2006; 41:e41–e43.17. Sekaran P, Shawis R. Perineal groove: a rare congenital abnormality of failure of fusion of the perineal raphe and discussion of its embryological origin. Clin Anat. 2009; 22:823–825.18. Masumoto H, Takenaka A, Rodríguez-Vázquez JF, Murakami G, Matsubara A. Reappraisal of intergender differences in the urethral striated sphincter explains why a completely circular arrangement is difficult in females: a histological study using human fetuses. Anat Cell Biol. 2012; 45:79–85.19. Murphy F, Puri P, Hutson JM, Holschneider AM. Incidence and frequency of different types, and classification of anorectal malformations. In : Holschneider AM, Hutson JM, editors. Anorectal Malformations in Children. Berlin: Springer;2006. p. 163–184. .20. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 2011; 193:500–508.21. Radlanski RJ, Renz H, Klarkowski MC. Prenatal development of the human mandible. 3D reconstructions, morphometry and bone remodelling pattern, sizes 12-117 mm CRL. Anat Embryol (Berl). 2003; 207:221–232.22. Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S, Sánchez-Montesinos I, Mérida-Velasco JA. Morphogenesis of the second pharyngeal arch cartilage (Reichert's cartilage) in human embryos. J Anat. 2006; 208:179–189.23. Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation). Okajimas Folia Anat Jpn. 2010; 87:49–58.24. Arakawa T, Hwang SE, Kim JH, Wilting J, Rodríguez-Vázquez JF, Murakami G, Hwang HP, Cho BH. Fetal growth of the anal sinus and sphincters, especially in relation to anal anomalies. Int J Colorectal Dis. 2016; 31:493–502.25. Hirata E, Fujiwara H, Hayashi S, Ohtsuka A, Abe S, Murakami G, Kudo Y. Intergender differences in histological architecture of the fascia pelvis parietalis: a cadaveric study. Clin Anat. 2011; 24:469–477.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Median Raphe Cyst in a 2-Year-Old Boy
- A Case of Perineal Hemangioma, External Genitalia Malformations, Lipomyelomeningocele, Vesicorenal Abnormalities, Imperforate Anus, and Skin Tag (PELVIS) Syndrome with Extensive Perineal Infantile Hemangioma
- Median Raphe Cyst of the Penis
- A Case of Median Raphe Canal of the Penis
- Median Raphe Canal of the Penis and the Scrotum